The lecture below can be accessed on the Disease Management section of the Cleveland Clinic, under Allergy and Immunology (to go to this link and see others in the series, please click here)
Pericardial Disease
Dermot Phelan
Patrick Collier
Richard A. Grimm
Published: July 2015
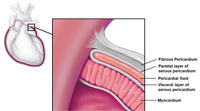
The pericardium is a thin fibroelastic sac composed of two layers that separate the heart from the surrounding mediastinal structures. The outer layer of the pericardium is referred to as the fibrous pericardium and usually measures less than 2 mm in thickness. The inner portion of the pericardium is a two-layered sac called the serous pericardium. The visceral pericardium or epicardium is closely adherent to the underlying myocardium and is reflected upon itself to form the outer parietal pericardium which lines the fibrous sac. Between the two layers of the serous pericardium lies the pericardial cavity which normally contains up to 50 mL of pericardial fluid (Figure 1). While the pericardium is not critical for life it serves important functions including maintenance of cardiac position within the chest and as a barrier to infection and inflammation.1
Acute Pericarditis
Definition
Acute pericarditis is an inflammatory process involving the pericardium that results in a clinical syndrome characterized by chest pain, pericardial friction rub, changes in the electrocardiogram (ECG) and occasionally, a pericardial effusion.2 Generally, the diagnosis requires 2 of these 3 features.
Prevalence
Acute pericarditis is the admitting diagnosis in 0.1% of hospital admissions and 5% of admissions with chest pain. It occurs more commonly in men than in women.
Etiology
The most common form of acute pericarditis is idiopathic, which accounts for about 90% of cases (Box 1). Other common causes include infection, renal failure, myocardial infarction (MI), post-cardiac injury syndrome, malignancy, radiation, and trauma.1 These are discussed in more detail later.
| Box 1: Common Causes of Pericarditis and Pericardial Effusion |
|---|
| Idiopathic |
Infectious
|
| Post-Myocardial infarction (early and late) |
| Postoperatively after open heart surgery |
Chest trauma
|
| Radiation |
Malignancy
|
Collagen vascular diseases
|
Inflammatory/infiltrative diseases
|
Metabolic
|
Pharmacologic
|
Signs and Symptoms
Acute pericarditis typically presents with acute onset severe, sharp retrosternal chest pain, often radiating to the neck, shoulders, or back. Positional changes are characteristic with worsening of the pain in the supine position and with inspiration; and improvement with sitting upright and leaning forward.
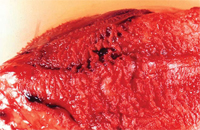
Classically, a scratchy, grating, high-pitched friction rub (which has been likened to the squeak of leather of a new saddle) is heard. This is felt to be caused by fibrinous deposits in the inflamed pericardial space (Figure 2) the timing of which can be mono-, bi-, or triphasic (corresponding to atrial systole, ventricular systole, and early ventricular diastole, respectively). It is best heard during inspiration at the left lower sternal border, with the patient leaning forward. The rub may disappear with the development of an effusion and impending cardiac tamponade.2
Specific Types
Idiopathic Pericarditis
The cause of acute pericarditis is often difficult to establish, and idiopathic pericarditis remains the most common diagnosis. Most cases are presumed to have a viral etiology.
Viral Pericarditis
Coxsackievirus B and echovirus are the most common viruses, and a fourfold increase in antiviral titers is required for the diagnosis. Patients often experience a prodrome of an upper respiratory tract infection. The prognosis of viral pericarditis is generally good, with a self-limited course, and if uncomplicated, patients may be treated on an outpatient basis.
Purulent Pericarditis
Before the antibiotic era, pneumonia was the prime cause of purulent pericarditis. Currently, causes include thoracic surgery, chemotherapy, immunosuppression, and hemodialysis, as well as extension from pneumonia and empyema. Presentation is usually acute with high fevers, chills, night sweats, and dyspnea, but the classic findings of chest pain or friction rub are rare. Cardiac tamponade is common (42% to 77% of patients in selected series), and mortality is high.
If purulent pericarditis is suspected, hospital admission, treatment with intravenous, broad-spectrum antibiotics and urgent drainage is recommended. Findings on pericardial fluid analysis include a high protein level (>6 g/dL), a low glucose level (<35 mg/dL), and a very high leukocyte count (6,000–240,000/mm3).3
Tuberculous Pericarditis
Tuberculous pericarditis occurs in 1% to 2% of cases of pulmonary tuberculosis. It remains the leading cause of pericarditis in some developing countries. Immunocompromised or human immunodeficiency virus (HIV)‑positive patients are at increased risk.4 Nonspecific symptoms such as dyspnea, fever, chills, and night sweats develop slowly, and a friction rub or chest pain is often absent. A patient with suspected or diagnosed pericardial tuberculosis should be hospitalized and antituberculous therapy started promptly.
Analysis of the pericardial fluid shows high specific gravity, very high protein level (often >6 g/dL), and predominantly lymphocytic cells. A pericardial biopsy with acid-fast bacilli polymerase chain reaction testing is recommended for all patients with suspected tuberculous pericarditis. However, a normal pericardial biopsy does not exclude the diagnosis.
Uremic and Dialysis-Associated Pericarditis
Uremic pericarditis occurs in 6% to 10% of patients with advanced renal failure before hemodialysis is initiated; blood urea nitrogen levels usually exceed 60 mg/dL. The typical ST-segment elevation on the ECG usually is absent. A large hemorrhagic effusion facilitated by impaired platelet function may occur, although tamponade is rare. Alternatively, a serous pericardial effusion related to fluid overload may occur. With both forms, initiation or intensification of hemodialysis is indicated, usually leading to improvement in 1 to 2 weeks.5,6
Pericarditis Following Myocardial Infarction
Post-MI pericarditis is a common complication (25%–40% of patients with MI) and occurs early, within 3 to 10 days after the MI. Its development correlates with the extent of necrosis, is more common with anterior than inferior infarcts, and is associated with a higher 1-year mortality rate as well as a higher incidence of congestive heart failure.7
The diagnosis of post-MI pericarditis requires symptoms or a new pericardial friction rub; a pericardial effusion alone is nonspecific. In addition to the typical ST elevation seen with acute pericarditis that may be difficult to differentiate from the actual MI in this setting, findings on the ECG are persistently positive T waves more than 2 days after MI or normalization of previously inverted T waves.8
Post-Cardiac Injury Syndrome
Dressler’s syndrome typically occurs 2 to 3 weeks after MI or open heart surgery. An autoimmune component involving sensitization to myocardial self-antigens at the time of infarction is believed to be responsible. The fully expressed syndrome consists of pleuritic chest pain, fever, leukocytosis, and a pericardial friction rub. Pleural effusions or pulmonary infiltrates may be seen.9
Malignancy
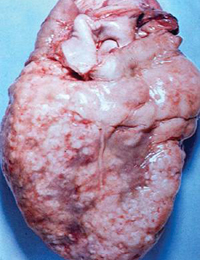
Pericarditis associated with malignancy is caused mainly by metastatic disease. Pericarditis is common in metastasized bronchogenic or breast carcinoma, Hodgkin’s disease, and lymphoma (Figure 3); it is rare in primary mesothelioma and angiosarcoma. Diagnosis is based on analysis of pericardial fluid cytology, which has a sensitivity ranging from 70% to 90% and a specificity of 95% to 100%.2
Radiation Pericarditis
Recent or remote mediastinal radiation can cause pericarditis at any time from weeks to months after the exposure.
Traumatic Pericarditis
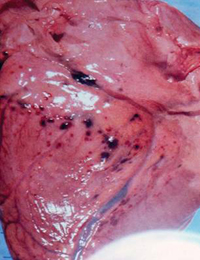
Sharp or blunt trauma (Figures 4 and 5) and invasive cardiac procedures such as electrophysiological ablation procedures, cardiac diagnostic, or interventional catheterization have been associated with pericardial irritation and inflammation.
Diagnosis
The diagnosis of acute pericarditis remains a clinical one based on history, physical examination, ECG and the echocardiogram. Other imaging studies, including computed tomography (CT) and magnetic resonance imaging (MRI) may be used in selected cases to investigate the pericardium.10
Electrocardiography
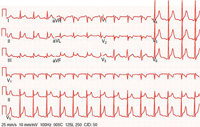
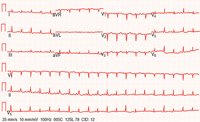
The ECG in acute pericarditis has four consecutive stages (Table 1). Stage 1, characterized by diffuse concave-upwards ST elevation and PR segment deviation in the direction opposite from the P polarity, is the most useful stage for the diagnosis of acute pericarditis (Figure 6). The distinction between pericarditis and acute MI is difficult at times, but there exist several helpful electrocardiographic clues (Table 2).11 Troponin levels may be elevated in up to 50% of patients with pericarditis but in the absence of myocarditis, the prognosis remains unchanged.12
Table 1: Stages of Acute Pericarditis by Electrocardiography
| Stage | Time | ST Segment | T Wave | PR Segment |
|---|---|---|---|---|
| 1 | Hours | Diffuse elevation | Upright | Leads aVR, V1: elevation All others: depression |
| 2 | Days | Resolution | Flattening | Resolution |
| 3 | Days-weeks | — | Inversion | — |
| 4 | Days-weeks | — | Upright | — |
Table 2: Electrocardiographic Differentiation of Acute Pericarditis and Myocardial Infarction
| Parameter | Acute Pericarditis | Acute Myocardial Infarction |
|---|---|---|
| ST elevation | Diffuse in I, II, and III Originating from S wave Concave upwards Lead V6: ST-T amplitude >0.24 mm |
Focal—vascular territory Originating from R wave Convex upwards |
| ST depression | Lead aVR only | Present; reciprocal changes to ST elevation according to territory |
| PR segment | Leads aVR, V1: elevation frequent | Rare changes if atrial infarction is present |
Blood Tests
Testing should include a complete blood count, markers of inflammation including erythrocyte sedimentation rate and/or C-reactive protein, markers of myocardial ischemia (troponins/CK-MB) to assess for myopericarditis, and assessment of renal function. Depending on presentation and clinical evaluation, additional blood testing may be necessary such as viral titers, blood cultures, thyroid-stimulating hormone, antinuclear antibodies, HIV serology, or QuantiFERON-TB assay.
Chest Radiography
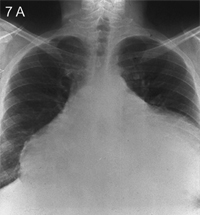
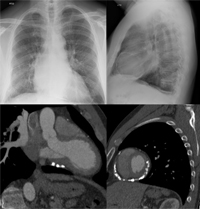
The chest radiograph may be entirely normal unless there is a pericardial effusion causing cardiomegaly (Figure 7A-B) or there are changes caused by an underlying disease.
Echocardiography
Trans-thoracic echocardiography (TTE) is used to detect and evaluate for pericardial effusion and any concomitant cardiac disease. Assessment of regional wall motion abnormalities can help differentiate acute pericarditis from myocardial ischemia. Echocardiography is essential in the presence of hemodynamic abnormalities, history of recent cardiac surgery, or if there is a clinical suspicion of a large or increasing pericardial effusion.
Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging
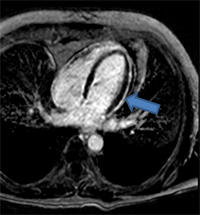
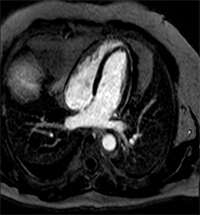
Advanced imaging modalities are increasingly used in the diagnotic assessment of patients with acute pericarditis, and to help guide their management. Cardiac computed tomography (CCT) is useful to assess for loculated effusions and to evaluate for increased thickness of the pericardium, although these features are not diagnostic of acute pericarditis. If there is clinical suspicion, CCT is useful in diagnosing the underlying etiology of acute pericarditis such as in the assessment of suspected malignancy-induced pericarditis. Inflammation of the pericardium produces characteristic changes that can be detected on cardiac magnetic resonance (CMR) imaging with very high sensitivity. Specifically, T2 weighted short-tau inversion recovery (STIR) imaging is useful for visualizing edema, while detection of a bright pericardium on late gadolinium enhancement (LGE) imaging is highly sensitive for pericardial inflammation (Figure 8A-B).1,10,13
Treatment
Most cases of acute pericarditis are uncomplicated and self-limited, and may be treated on an outpatient basis. Indications for an advanced imaging modality, hospital admission, or both include clinical suspicion of a large effusion, hemodynamic instability, severe pain or other symptoms, suspicion of a serious underlying condition, or any other signs or symptoms of clinical instability or impending deterioration. Other features which suggest a more complex course include fever >38°C, subacute onset, lack of response to at least one week of anti-inflammatory therapy, myopericarditis, immunosuppression, trauma, or concurrent treatment with oral anticoagulant therapy.11
Medical Management
In acute idiopathic and viral pericarditis, the aim of treatment is the resolution of pain and inflammation. For other etiologies of acute pericarditis treatment of the underlying disease is the mainstay of therapy.12,14 Combination therapy with colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended. Ibuprofen 600 mg or indomethacin 50 mg every 8 hours for one to two weeks followed by a gradual taper until resolution of symptoms and inflammatory markers, in combination with colchicine 0.6 mg twice daily (0.6 mg once daily if <70 kg) for 3 months is our standard recommendation.
Non-aspirin NSAIDs are contraindicated in the early period (<7–10 days) after MI (can predispose to cardiac rupture), and aspirin should be used instead (650–1,000 mg three times daily). Due to the risk of gastrointestinal toxicity, addition of gastrointestinal protection with a proton pump inhibitor is recommended in higher risk patient.
The randomized, open-label COPE (COlchicine for Acute PEricarditis) trial found that colchicine, in addition to aspirin, reduced the recurrence of pericarditis from 32.3% to 10.7% (P = 0.003).15 In the more recent randomized, double-blinded, placebo-controlled ICAP (Investigation on Colchicine for Acute Pericarditis) trial, a similar reduction in recurrent pericarditis was observed when colchicine was added to aspirin monotherapy (from 37.5% to 16.7%, P < 0.001). Unless their use is mandatory, anticoagulants should be avoided during the acute phase of pericarditis to reduce the risks of intra-pericardial bleeding and tamponade.
Treatment with glucocorticoids should only be considered in recurrent pericarditis for patients with symptoms refractory to standard therapy, or in immune-mediated or uremic pericarditis.16 Use of steroids in acute pericarditis has been associated with recurrence.17 The correct dosing for steroid therapy remains controversial; however, our practice it to being with approximately 0.5 mg/kg/day with a prolonged and slow taper. As with non-aspirin NSAIDs, steroids should be avoided in post-MI pericarditis due to their impairment of scar formation and a consequently increased incidence of myocardial wall rupture.
Pericardiectomy
Pericardiectomy is not indicated in the management of acute pericarditis.
Outcomes
Patients with uncomplicated acute pericarditis should have regular follow-up after the initial visit to ensure resolution of symptoms and rule out the development of constrictive symptoms.
Pericardial Effusion
Definition
Pericardial effusion is defined as an increased amount of pericardial fluid.
Etiology
The etiology of a pericardial effusion can often be deduced from the clinical presentation (Box 1); for example, in patients who present with severe hypothyroidism, end-stage kidney failure, acute myocardial infarction, or who underwent a recent invasive cardiac procedure. The relative frequency of different etiologies of pericardial effusions depends on the geography and the patient population.18,19
Pathophysiology
The pericardial sac normally contains up to 50 mL of fluid; it can hold 80 to 200 mL of fluid acutely, and even up to 2 L if the fluid accumulates slowly.
Signs and Symptoms
Pericardial effusions may be asymptomatic unless associated with inflammation or tamponade physiology. Other symptoms arise from the compression of surrounding structures (lung, stomach, phrenic nerve) or diastolic heart failure and include chest pressure or pain, dyspnea, nausea, abdominal fullness, and dysphagia. Phrenic nerve irritation can cause hiccups.
With a small effusion, the physical examination is unremarkable. Larger effusions cause muffled heart sounds and, rarely, Ewart’s sign (dullness to percussion, bronchial breath sounds, and egophony below the angle of the left scapula).
Diagnosis
Electrocardiography
Low voltage and electrical alternans (Figure 9) may be seen if the effusion is large — the former due to increased distance from the chest leads, the latter caused by swinging of the heart within the effusion. These features in combination with sinus tachycardia should raise concern regarding the potential hemodynamic impact of the effusion and urgent assessment for tamponade physiology should be performed. Absence of hypotension should not dissuade the clinician from a possible diagnosis of pericardial effusion and tamponade, because hemodynamic instability usually does not occur until the patient nears circulatory collapse.
Chest Radiography
Cardiomegaly occurs if there is more than 250 mL of fluid in the pericardial sac (Figure 7A). Displacement of the pericardial lining more than 2 mm away from the lower heart border is best seen on lateral film.20
Echocardiography
Echocardiography is the main diagnostic tool used in the evaluation of pericardial effusion. A pericardial effusion causes an echo-free space between visceral and parietal pericardium; the extent of the space defines the size of the effusion (Table 3). Large effusions can produce the picture of a swinging heart. Although echocardiography is the imaging modality of choice for diagnosing a pericardial effusion, small loculated effusions pose a greater diagnostic challenge.
Table 3: Sizing of Pericardial Effusion by Echocardiography
| Parameter | Small | Medium | Large |
|---|---|---|---|
| Volume (mL) | <100 | 100-500 | >500 |
| Localization | Localized | Circumferential | Circumferential |
| Width (cm) | <1 | 1-2 | >2 |
Magnetic Resonance Imaging and Computed Tomography
If there is a high level of clinical suspicion of a pericardial effusion and the TTE is nondiagnostic, alternative tomographic imaging should be considered. This is especially true for patients who have recently undergone open heart surgery, who frequently have echocardiographic studies with suboptimal imaging quality, and effusions which may be loculated and complex. Cardiac CT and MRI will also aid in differentiating an effusion from pericardial fat, pericardial cysts and pleural effusions, all of which can mimic a pericardial effusion.
Laboratory Tests
Laboratory analysis in a patient with a pericardial effusion should include a complete blood count, chemistry panel, and erythrocyte sedimentation rate. Further testing should be done according to clinical suspicion.
Analysis of Pericardial Fluid
The initial inspection should assess whether the fluid is hemorrhagic, purulent, or chylous. A red blood cell count higher than 100,000/mm3 is suggestive of trauma, malignancy, or pulmonary embolism (rare). Chylous fluid implies injury to the thoracic duct by trauma or infiltration. The fluid should be sent for a cell count; Gram stain; culture; cytology; acid-fast bacilli; determination of glucose, protein, and lactate dehydrogenase (LDH) levels; and specific gravity. The parameters listed in Table 4 have a high sensitivity for differentiating exudates versus transudates. An elevated protein level higher than 6.0 g/dL often indicates tuberculous, purulent, or parapneumonic effusion. An isolated increased fluid LDH level (>300 U/dL) with a normal serum LDH level is most likely caused by malignancy. A low pericardial fluid glucose level (<60–80 mg/dL) may be caused by parapneumonic, rheumatoid, tuberculous, or malignant effusion. However, no diagnostic test of pericardial fluid is specific for effusion associated with postpericardiotomy syndrome, radiation or uremic pericarditis, hypothyroidism, or trauma. The overall diagnostic yield of pericardial fluid analysis and biopsy is low (about 20%), emphasizing the importance of clinical history and examination.3
Table 4: Pericardial Effusion: Exudate Versus Transudate
| Parameter | Exudate | Transudate |
|---|---|---|
| Cause | Malignancy | Radiation |
| Infectious, parainfectious | Uremia | |
| Postpericardiotomy syndrome | Hypothyroidism | |
| Collagen vascular disease | Trauma | |
| Specific gravity (g/mL) | >1.015 | <1.015 |
| Total protein (g/dL) | >3.0 | <3.0 |
| Fluid-to-serum protein ratio | >0.5 | <0.5 |
| Fluid-to-serum LDH ratio | >0.6 | <0.6 |
| Fluid-to-serum glucose ratio | <1.0 | >1.0 |
LDH = lactate dehydrogenase.
Treatment
The medical management of pericardial effusion is based on treating the underlying cause. Effusions causing pretamponade or tamponade require immediate drainage. Volume expansion and inotropic support may be used for hemodynamic stabilization pending drainage. In the immediate postoperative setting, surgical management and open drainage are preferred because of the high incidence of loculated effusions. Anti-coagulation should be avoided if possible.
Pericardiocentesis
Pericardiocentesis should be performed for diagnostic purposes if the cause of the effusion is unclear, or if malignancy or a purulent effusion is suspected. Therapeutic pericardiocentesis should be performed for effusions amenable to percutaneous drainage that are causing pretamponade or tamponade physiology.
Surgical Treatment
Subxiphoid pericardiostomy, also known as a pericardial window, may be done under local anesthesia. It has a high success rate, with few complications, and recurrence of fluid accumulation is rare.
Percutaneous balloon pericardiotomy is the least invasive of the surgical procedures. It is used primarily as palliative treatment for neoplastic effusions with a poor prognosis. The success rate for relieving reaccumulation of pericardial fluid is 85% to 92% at 30 days. It may be performed in the catheterization laboratory under fluoroscopy using a balloon-dilating catheter.
Outcomes
After drainage, follow-up echocardiography should be performed in all patients to rule out reaccumulation and/or constrictive physiology.
Cardiac Tamponade
Definition
Cardiac tamponade occurs when fluid accumulation in the finite serous pericardial space causes an increase in pressure, with subsequent cardiac compression and hemodynamic compromise.
Prevalence
Of patients with large pericardial effusions, 25% to 30% develop tamponade.21
Pathophysiology
Elevated intrapericardial pressure leads to progressive limitation of diastolic ventricular filling, resulting in lowered cardiac output.18,19
Signs and Symptoms
Symptoms
Symptoms resulting from decreased cardiac output and congestion include dyspnea, chest discomfort, weakness, restlessness, agitation, drowsiness, oliguria, and anorexia. If the tamponade develops acutely as a complication of an acute MI (free wall rupture) or trauma, the presentation is usually catastrophic, with shock or sudden death.
Physical Examination Findings
The combination of the classic findings known as Beck’s triad (hypotension, jugular venous distention, and muffled heart sounds) occurs in only 10% to 40% of patients. Tachycardia and tachypnea are common. Pulsus paradoxus is defined as an inspiratory decline in systolic blood pressure of more than 10 mmHg resulting from compression and poor filling of the left ventricle. Pulsus paradoxus is nonspecific and insensitive and can occur with extracardiac disease, such as severe chronic obstructive pulmonary disease or asthma.22
Diagnosis
The diagnosis of cardiac tamponade is a clinical diagnosis with echocardiagraphic comfirmation.
Electrocardiography
The ECG may be unremarkable. Abnormal findings on ECG include tachycardia, electrical alternans (Figure 9), low voltage, and may include changes associated with acute pericarditis (Figure 6).
Transthoracic Echocardiography
Usually, a moderate-size or large pericardial effusion is present and leads to increasing compression and subsequent diastolic compression of the cardiac chambers, usually in the sequence right atrium, right ventricle, left atrium (with the lowest pressure chamber being affected first). The most sensitive finding for tamponade physiology on the echocardiogram is inferior vena cava plethora, with absent inspiratory collapse; however this is not very specific. Right atrial inversion for >1/3 the cardiac cycle length is the most sensitive and specific sign. Right ventricular diastolic inversion may also been seen. Other, less-specific findings include excessive respiratory variations across the mitral valve inflow (>30%), which is analogous to pulsus paradoxus and the tricuspid inflow (>60%). Absent diastolic flow from the hepatic views suggests tamponade physiology.1,19
Right Heart Catheterization
The most typical finding on right heart catheterization is equalization of mean right atrial, right ventricular and pulmonary artery diastolic, and mean pulmonary capillary wedge pressures.
Differential Diagnosis
The symptoms of pericardial tamponade can mimic those of right-sided heart failure, right ventricular infarction, constrictive pericarditis, and pulmonary embolism. However, with the use of echocardiography and occasionally right heart catheterization, these may be distinguished.
Treatment
Patients with pretamponade and tamponade require immediate hospital admission and prompt pericardial drainage by pericardiocentesis. The drain catheter may be left in place for up to 48 hours if drainage is slow or reaccumulation likely. If follow-up echocardiography documents fluid reaccumulation, a pericardial window should be considered, because the infection risk associated with a pericardial drain increases after 48 hours.19 Pending drainage, intravenous fluid expansion and inotropic support may be used for hemodynamically unstable patients.
Pericardial Constriction
Definition
Constrictive pericarditis refers to an abnormal scarring and loss of elasticity of the pericardium, resulting in impaired ventricular filling and decreased cardiac output.
Etiology
The frequency of different causes of constrictive pericarditis depends on the population and geography in question. In developed countries, cardiac surgery and idiopathic constriction are the leading cause, while in certain developing countries tuberculous remains the number one etiology.
Pathophysiology
The initiating event results in a chronic inflammatory pericardial process, resulting in fibrinous scarring and occasionally calcification of the pericardium (Figure 10). As the heart becomes encased with a non-compliant pericardium, ventricular interdependance and dissociation between the intrathoracic and intracardiac pressure occurs. In constriction, normal expansion of the right heart is restricted by the pericardium and the septum shifts to the left to accommodate the increase in venous return with inspiration; the opposite movement occurs with expiration. Normally, there is a reduction in intrathoracic and intracardiac pressure with inspiration. The rigid pericardium impedes normal ventricular expansion and therefore venous return and pulmonary venous pressure. Because intrathoracic pressure drops but left ventricular pressure does not decrease with inspiration there is a reduction in the transpulmonary gradient. This leads to impaired ventricular filling and decreased cardiac output. Ultimately, right and then left ventricular heart failure develop. Distinguishing heart failure caused by constrictive physiology from diastolic restrictive physiology is a classic diagnostic dilemma.
Signs and Symptoms
Clinical Symptoms
Symptoms are often vague and their onset is insidious; they include malaise, fatigue, and decreased exercise tolerance. With progression of constrictive pericarditis, symptoms of right-sided heart failure (eg, peripheral edema, nausea, abdominal discomfort, ascites) become apparent and usually precede signs of left-sided failure (eg, exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea).
Physical Examination Findings
Increased ventricular filling pressures cause jugular venous distention and Kussmaul’s sign (paradoxical rise in jugular venous pressure on inspiration), which is sensitive but not specific for constrictive pericarditis.23 Auscultation may reveal muffled heart sounds and occasionally a characteristic pericardial knock (60–200 msec after the second heart sound), which is caused by sudden termination of ventricular inflow by the encasing pericardium.
Constrictive effusive pericarditis consists of a tense pericardial effusion in the presence of pericardial constriction, and both tamponade and constrictive signs and symptoms are present.
Diagnosis
Electrocardiography
The ECG does not show specific findings, but low voltage may be seen.
Laboratory Test Findings
Brain natriuretic peptide (BNP) is a serum biomarker that can help distinguish constrictive pericarditis from restrictive cardiomyopathy. Despite elevated filling pressures in both conditions, levels of BNP are significantly higher in restrictive cardiomyopathy.24
Chest Radiography
Pericardial calcifications (Figure 10), pleural effusions, and biatrial enlargement may be noted on the chest radiograph.
Echocardiography
Echocardiography is the best imaging modality for assessing hemodynamic parameters noninvasively. M-mode echocardiography is useful for looking for rapid motion followed by abrupt flattening of the left ventricular free wall in early and mid diastole respectively. Two-dimensional echocardiography may demonstrate a thickened pericardium (about one third of cases), myocardial tethering, abrupt cessation of left ventricular and right ventricular diastolic filling, biatrial enlargement, tubular deformity of the left ventricle, respirophasic septal shift, septal bounce and inferior vena cava plethora with absent inspiratory collapse. Doppler echocardiographic findings have the highest sensitivity and specificity for detecting constrictive physiology. Excessive respiratory variations in transmitral, transtricuspid, pulmonary venous, and hepatic vein flow are characteristic. Reduced lateral annular diastolic velocity (e’) compared to normal/increased velocity of the septal/medial annular velocity is termed annulus reversus and is a typical feature. Low tissue velocity at both medial and lateral annuli suggests restriction. More recently developed echocardiographic modalities such as strain imaging have enhanced the ability to discriminate between restriction and constriction.25
Right Heart Catheterization
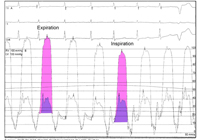
Direct pressure measurements are performed if there is doubt about the diagnosis. Characteristic features in the right atrium include: elevated right atrial pressures, prominent x and y decents and Kussmaul’s sign. Square-root or dip-and-plateau right ventricular pressure waveforms reflect impaired ventricular filling. Because of the fixed and limited space within the stiff pericardium, end-diastolic pressure equalization (typically within 5 mmHg) occurs between these cardiac chambers. Pulmonary artery systolic pressures are usually normal in pericardial constriction; higher pulmonary pressures suggest a restrictive cardiomyopathy. The ratio of the right ventricular to left ventricular systolic pressure-time area during inspiration compared to expiration is a highly sensitive and specific means of differentiating constriction from restriction (Figure 11).
Magnetic Resonance Imaging and Computed Tomography
Computed tomography is the imaging modality of choice to evaluate the thickness of the pericardium and for pericardial calcification. Although the finding of thickened pericardium on CT is specific for constriction, up to 18% of patients with constriction confirmed by other modalities do not have pericardial thickening.26 Computed tomography can also assist surgical planning.
While echocardiography is the first choice imaging modality for assesssment of constriction, for many patients CMR is becoming increasingly utilized in the initial evaluation, particularly if any ambiguity remains regarding the diagnosis, if there is suggestion of active inflammation, or if the duration of symptoms has been brief. Cardiac magnetic resonance is very useful to differentiate a small pericardial effusion from pericardial thickening. The superior signal-to-noise and contrast-to-noise ratio of CMR allows precise evaluation of the morphological and hemodynamic changes seen in pericardial constriction. Real-time cine sequences allow evaluation of the features described above in the 2D echocardiographic evaluation of pericardial constriction, which is useful if echocardiographic images are sub-optimal. Phase encoding velocity imaging potentially provides similar data to Doppler echocardiography but is not yet generally employed in routine practice. The main advantages of CMR over echo are the STIR and LGE sequences which permit evaluation of edema and inflammation that can impact management decisions.27
Treatment
The degree of constrictive physiology occurs along a spectrum of severity. Early forms may be difficult to diagnose without a high degree of clinical suspicion. It is increasingly recognized that some of these patients may respond to medical therapy, without surgical intervention; this is referred to as transient constrictive pericarditis.28 Inflammation seen on LGE CMR images is a good predictor of those patients that may respond to anti-inflammatory therapy.27
Medical treatment is limited in chronic constrictive pericarditis in the absence of active inflammation. Diuretics and a low-sodium diet may be tried for patients with mild to moderate (New York Heart Association Class I or II) heart failure symptoms or contraindications to surgery.14 For patients that do not respond to medical treatment, pericardiectomy is advised.
For effusive-constrictive pericarditis therapy includes pericardiocentesis initially, followed by treatment with anti-inflammatory agents. Frequently, pericardiectomy is necessary for long-term management.29
Outcomes
Recurrence following surgery is caused mainly by incomplete resection of the pericardium. Without surgical treatment, biventricular failure develops. Long-term survival after pericardiectomy is worse than matched controls but this is mainly related to the underlying etiology.30,31
Summary
Acute Pericarditis
- Acute pericarditis manifests with acute positional and pleuritic chest pain, ECG changes, is sometimes accompanied by a pericardial rub, and is often associated with pericardial effusion.
- In the United States, 90% of cases of acute pericarditis are idiopathic.
- Non-aspirin NSAIDs and colchicine should be used for most patients, except for those with post-MI pericarditis (they should receive aspirin and colchicine).
- Colchicine reduces the recurrence of pericarditis.
- Steroids can increase the recurrence rate of pericarditis and the risk of acute myocardial rupture. They should be reserved for cases where steroids are indicated to treat the underlying cause of the effusion, or for recurrent/resistant pericarditis.
Pericardial Effusion and Cardiac Tamponade
- The pericardial sac normally contains up to 50 mL of fluid; it can hold 80 to 200 mL of fluid acutely, and even up to 2 L if the fluid accumulates slowly.
- There are a wide range of medical conditions that can precipitate a pericardial effusion; the etiology can often be deduced from the clinical presentation. In the absence of hemodynamic compromise, treatment of the underlying medical condition is indicated.
- Cardiac tamponade is a clinical diagnosis made by documenting one or more of the following in the presence of a pericardial effusion: tachycardia, pulsus paradoxus (>10 mmHg inspiratory decline in systolic BP), jugular venous distention, and muffled heart sounds.
- The signs depend on the volume of fluid in the pericardial sac and the rate at which the fluid accumulates.
- Echocardiography can confirm the diagnosis at an early stage and help with the drainage of the effusion.
- Pericardiocentesis should be performed for diagnostic purposes if the cause is unclear or if purulent infection is suspected. Therapeutic pericardiocentesis should be performed if there is evidence of hemodynamic compromise.
Constrictive Pericarditis
- Constrictive pericarditis results from an abnormal scarring of the pericardium that causes impairment of diastolic filling.
- A diagnostic challenge often lies in differentiating constriction from restriction. The diagnosis can be made by noting hemodynamic derangements on echocardiography/CMR/right heart catheterization and a thickening of the pericardium on echo/CT/MRI.
- About 20% of patients do not demonstrate thickening of the pericardium by CT.
- For patients that do not respond to medical therapy, the treatment of choice is surgical excision of the pericardium.
Suggested Readings
- Blanchard DG. Pericardial effusion and AIDS. Circulation 1996; 94:2312.
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med 2004; 351:2195–2202.
- Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald’s Heart Disease A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005.
References
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26:965–1012.e15.
- LeWinter MM. Pericardial diseases. In: Libby P, Bonow RO, Mann DL, Zipes DP, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, Single Volume. 8th ed. Philadelphia, PA: Saunders; 2007:1829–1853.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
- Fowler NO, Tuberculous pericarditis. JAMA 1991; 266:99–103.
- Rostand SG, Rutsky EA. Pericarditis in end-stage renal disease. Cardiol Clin 1990; 8:701–707.
- Wood JE, Mahnensmith RL. Pericarditis associated with renal failure: evolution and management. Semin Dial 2001; 14: p. 61–66.
- Sugiura T, Iwasaka T, Takayama Y, et al. Factors associated with pericardial effusion in acute Q wave myocardial infarction. Circulation 1990; 81:477–481.
- Oliva PB, Hammill SC, Edwards WD. Electrocardiographic diagnosis of postinfarction regional pericarditis. Ancillary observations regarding the effect of reperfusion on the rapidity and amplitude of T wave inversion after acute myocardial infarction. Circulation 1993; 88:896–904.
- Khan AH. The postcardiac injury syndromes. Clin Cardiol 1992; 15:67–72.
- Bogaert J, Francone M. Pericardial disease: value of CT and MR imaging. Radiology 2013; 267:340–356.
- Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y. Controversial issues in the management of pericardial diseases. Circulation 2010; 121:916–928.
- Imazio M, Brucato A, Mayosi BM, et al. Medical therapy of pericardial diseases: part I: idiopathic and infectious pericarditis. J Cardiovasc Med (Hagerstown) 2010; 11:712–722.
- Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol 2006; 16:569–574.
- Imazio M, Brucato A, Mayosi BM, et al. Medical therapy of pericardial diseases: part II: noninfectious pericarditis, pericardial effusion and constrictive pericarditis. J Cardiovasc Med (Hagerstown) 2010; 11:785–794.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016.
- Maisch B, Seferović PM, Ristić AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Lotrionte M, Biondi-Zoccai G, Imazio M, et al. International collaborative systematic review of controlled clinical trials on pharmacologic treatments for acute pericarditis and its recurrences. Am Heart J 2010; 160:662–670.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J 2013; 34:1186–1197.
- Dunn PM, MacNichol J, Krekeler MM, Rotter SM, Brown K. Roentgenograms in pericardial effusion. JAMA 1987; 258:1890–1891.
- Corey GR, Campbell PT, Van Trigt P, et al. Etiology of large pericardial effusions. Am J Med 1993; 95:209–213.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Bilchick KC, Wise RA. Paradoxical physical findings described by Kussmaul: pulsus paradoxus and Kussmaul’s sign. Lancet 2002; 359:1940–1942.
- Leya FS, Arab D, Joyal D, et al. The efficacy of brain natriuretic peptide levels in differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Coll Cardiol 2005; 45:1900–1902.
- Kusunose K, Dahiya A, Popović ZB, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging 2013; 6:399–406.
- Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 2003; 108:1852–1857.
- Feng D, Glockner J, Kim K, et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation 2011; 124:1830–1837.
- Sagristà-Sauleda J, Permanyer-Miralda G, Candell-Riera J, Angel J, Soler-Soler J. Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol 1987; 59:961–966.
- Syed FF, Ntsekhe M, Mayosi BM, Oh JK. Effusive-constrictive pericarditis. Heart Fail Rev 2013.; 18:277–287.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028; discussion 1028.
- Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol 2004;271–275.