Psoriatic Arthritis
M. Elaine Husni
Published: August 2010
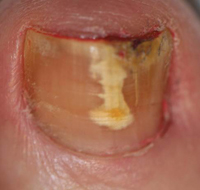
Psoriatic arthritis (PsA) is a unique type of inflammatory arthritis that is associated with skin psoriasis. There is increasing evidence on molecular, cellular, and tissue levels that PsA is a distinctive form of inflammatory arthritis compared with rheumatoid arthritis (RA), the most common form of inflammatory arthritis. Psoriatic arthritis can affect the joints and surrounding structures such as the tendons and ligaments, specifically as dactylitis and enthesitis. In addition to the psoriatic skin changes, it can affect the scalp and nails, causing pitting, ridging, and distal onycholysis. Prompt diagnosis and treatment can relieve pain and inflammation and possibly help prevent progressive joint involvement and damage.
Signs and Symptoms
PsA causes, pain, swelling, stiffness, and tenderness of the joints, which limits motion. The disease has a heterogenous presentation including monoarthritis, oligoarthritis, or polyarthritis. The most common peripheral joint involvement is in the distal interphalangeal joints; this is commonly associated with nail changes of that digit. PsA can also affect the lower back, knees, ankles, and wrists. It can be associated with fatigue and morning stiffness. This disease can also involve the spine and the sacroiliac joint either alone or in combination with peripheral disease.
The psoriatic skin disease usually precedes the joint symptoms. More than 60% to 70% of psoriasis patients present many years before joint symptoms occur, and about 10% to 15% of patients present with concomitant skin and joint symptoms. Skin psoriasis typically manifests with silvery or gray scaly patches on the extensor surfaces (elbows and knees), torso, lower spine, and scalp. Therefore, a careful patient history can also provide important diagnostic clues to this heterogeneous presentation. For example, a family history of psoriasis or prior history of skin rash might warrant greater scrutiny of hidden areas such as scalp, umbilicus, ears, and perianal areas.
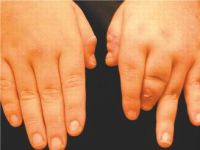
Because the clinical presentation can be varied, five different subtypes of PsA have been described and can overlap (Box 1). These include distal interphalangeal joint–predominant arthritis, symmetric polyarthritis-predominant arthritis, asymmetric oligoarthritis or monoarthritis, axial disease predominant spondylitis and/or sacroiliitis, and arthritis mutilans. Arthritis mutilans is a very rare, painful, and rapidly destructive type of PsA. This condition is characterized by deforming arthritis especially of the hands and by resorption of phalangeal bones (Figs. 1 and 2).
| Box 1 Subtypes of Psoriatic Arthritis |
|---|
| Distal interphalangeal joint–predominant arthritis (10%) |
| Symmetric polyarthritis-predominant arthritis (5%-20%) |
| Asymmetric oligoarthritis or monoarthritis (70%-80%) |
| Axial disease predominant spondylitis and/or sacroiliitis (5%-20%) |
| Arthritis mutilans (rare) |
Other distinctive clinical features include sausage-like swelling of the fingers and toes, called dactylitis. This can also be seen in patients with reactive arthritis conditions. Inflammation of the tendon sheath (tenosynovitis) or inflammation of the entheses (enthesitis) is also seen. Inflammation of the eye, such as conjunctivitis, can also be associated with PsA.
Prevalence, Risk Factors, and Natural History
The exact prevalence of PsA is unknown, but estimates vary from 0.3% to 1% of the U.S. population, with a reported prevalence of 7% to 42% in patients with psoriasis.1 Psoriatic arthritis can develop at any time, but it appears most often between the ages of 30 and 50. Unlike other types of inflammatory arthritis, which have a large female predominance, PsA seems to affect men at about the same or slightly higher rate compared to women.
The onset of PsA symptoms may vary. Typically, PsA manifests as a mild, oligoarticular disease, but it can become polyarticular with time and progresses to a severe, erosive condition in at least 20% of patients. Aggressive disease is seen more commonly in those who exhibit polyarticular or erosive PsA at presentation. Additional factors can predictive a worse prognosis for psoriatic patients. These include extensive skin involvement, strong family history of psoriasis, and disease onset before 20 years of age (Box 2).
| Box 2 Predictors of Worse Prognosis |
|---|
| Polyarticular or erosive disease at presentation |
| Association with extensive skin involvement |
| Strong family history of skin disease |
| Disease onset at younger than 20 years of age |
Pathophysiology
There appear to be multiple factors contributing to the pathogenesis of PsA, including genetic, environmental, and immunologic. The exact cause of PsA has not been identified, and the concomitant pathogenic connection between the skin and joints is not clear.
Genetic Factors
As many as 40% of people with PsA have a family history of skin or joint disease.2 Interestingly, children of parents with psoriasis are three times more likely to have psoriasis and are at greater risk for developing PsA than children born to parents without psoriasis. There are proposed major histocompatibility complex (MHC) and non-MHC loci that may be associated with increased susceptibility to PsA.
Environmental Factors
Bacterial and viral infections have been implicated as a cause or trigger in PsA. Some studies on psoriatic plaque have suggested enhanced humoral and cellular immunity to gram-positive bacteria; however, no direct relationship between bacteria and psoriasis has been proved. Another environmental trigger has been proposed in relation to the Koebner phenomenon, whereby arthritis can develop at sites of traumatized skin.3
Immunologic Factors
The most compelling pathogenic factors are the similar immunopathology of psoriasis and PsA. There is evidence that activated T cells are present in both skin and joint tissue. The success of anti–tumor necrosis factor α (TNF-α) therapy for both skin and joint symptoms of PsA patients has also provided new insights into the pathogenesis of PsA. It is likely that cytokines such as TNF-α are critically involved in guiding the inflammatory process, leading to cartilage and bone degradation as well skin inflammation.
Diagnosis
Diagnosing PsA is necessarily complex; the most critical factor is the absence of validated criteria for identifying and classifying cases and for diagnosing the disease. The standard rheumatologic laboratory tests such as rheumatoid factor and anti–cyclic citrullinated peptide (anti-CCP) and radiographic changes may be negative, adding to the difficulty of diagnosis.
PsA is mainly diagnosed by establishing the presence of characteristic signs and symptoms associated in both the skin and joints and by ruling out more common inflammatory arthritis. However, these clinical features are not confined to PsA, and PsA and RA share many common characteristics. Diagnosis may be easier to confirm if psoriasis coexists with symptoms of arthritis. However, in as many as 15% of cases, symptoms of PsA appear before symptoms of psoriasis (psoriatic arthritis sine psoriasis). A careful medical history, physical examination, blood tests, magnetic resonance imaging (MRI), and x-rays of the involved joints along with a dermatologic evaluation may be used to diagnose PsA.
It is important diagnose PsA early so that treatment can quickly relieve pain and inflammation and prevent irreversible joint damage. Without treatment, PsA is potentially disabling and crippling. In the very early stages of the disease, x-rays usually do not reveal signs of arthritis and might not help in making a diagnosis. In the later stages, x-rays can show changes that are characteristic of PsA, such as the pencil-in-cup sign, where the end of the bone gets whittled down to a sharp point. The most typical radiographic changes include erosive changes similar to those seen in RA (in the paramarginal rather than marginal areas) and new bone formation in the distal peripheral joints and at the enthesial sites. Additional x-ray changes include fluffy periostitis, ankylosis, and loss of bone at the distal phalanges. MRI can be more sensitive in detecting soft tissue inflammation as well as changes in the sacroiliac joints. However, most of the changes can occur in the later stages of the disease where clinical signs and symptoms for PsA have already been established.
Initially, most patients with PsA were thought to have mild, short-lived form of arthritis. However, it is now known that PsA can affect joints early in the disease course with irreversible joint damage, and skin psoriasis can severely decrease in a patient’s quality of life.
Treatment
The goals of treatment for PsA should include early diagnosis, with early aggressive treatment aimed at halting or minimizing joint damage and clearing the skin psoriasis. The treatment recommendations for PsA have been largely borrowed from other inflammatory arthritis treatment protocols such as RA trials. Thus, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and traditional disease-modifying antirheumatic drugs (DMARDs) have been based upon expert consensus among rheumatologists and dermatologists; only a limited number of evidence-based clinical trials exist for PsA patients. Optimal treatment of PsA should be targeted to both skin and joint disease, In addition, a subset of PsA patients require additional treatment for nail and scalp involvement and for dactylitis and enthesis involvement. There has been great interest in newer agents (such as biologic therapies) that may be used as monotherapy or in combination with traditional DMARDs to treat both the skin and joint symptoms of this condition.
Because PsA has such a heterogeneous presentation, treatment can usually be tailored to the predominant arthritis presentation. In general, treatment can be aimed at peripheral or axial predominance, skin and nail predominance, or dactylitis and enthesitis. Mild or limited joint disease might respond to initial treatment with NSAIDs; however, DMARDs are indicated in patients with more-aggressive and erosive disease.
Steroidal and Nonsteroidal Anti-inflammatory Therapy
Initial therapy for PsA involving peripheral and axial disease is NSAIDs. However, with limited studies in this population, it is also important to note that NSAIDs have not demonstrated disease modification but rather are shown to decrease pain scores and joint symptoms compared to placebo. There has also been some concern that NSAID therapy can exacerbate the skin disease,4 with the potential mechanism of shunting of arachidonic acid metabolites down the leukotriene pathway. Other studies have concluded this is not a substantial clinical problem.5
In general, steroid therapy has not been a mainstay of treatment because of the risk of provoking a pustular flare of the skin disease upon withdrawal. Occasionally, rheuamtologists have found intra-articular injections of corticosteroid very useful in the management of monoarthritis or oligoarthritis.
Traditional DMARD Therapy
The most widely used first-line DMARD agent that has demonstrated efficacy in a randomized clinical trial for both joint and skin disease for PsA is methotrexate. Although this drug is more widely studied in RA patients, methotrexate does have the longest duration of clinical experience in PsA patients. Overall, dosages of methotrexate can average a low weekly dosage of 5-10 mg, which can be increased as needed up to 20-25 mg per week. Patients on methotrexate do need to avoid alcohol because of the potential for liver abnormalities that can be associated with its use. Rheumatologists and dermatologists commonly use methotrexate for these conditions; however, the guidelines on long-erm monitoring and proper patient selection to minimize toxicity of this therapy differ among the subspecialties specifically in regard to the need for liver biopsy during treatment with methotrexate. Female patients attempting to conceive must avoid methotrexate as it has been demonstrated to cause birth defects.
Sulfasalazine has also been shown to be effictive in treating peripheral joint symptoms, although it has less effect on skin and axial disease symptoms in PsA.
Other traditional disease-modifying agents for inflammatory arthritis have been used in PsA patients, such as azathioprine, leflunomide, and cyclosporine. However, only very small controlled trials with limited numbers of PsA patients have been completed with these agents. These treatments have been generally reserved for patients who appear to be intolerant of the better-studied DMARDs, such as methotrexate or sulfasalazine.
Biological Response Modifiers
More-targeted biological therapy for autoimmune disease has made a significant improvement in the way we can treat PsA. With advancing knowledge in immunology and molecular biology, bioengineered proteins or small molecules have been developed to block very specific targets; these are called biologic response modifiers. The most efficacious biologic response modifiers specifically inhibit TNF-α, which has been implicated as the critical molecule responsible for mediating the progression of joint and skin damage in these patients.
Three TNF inhibitors are approved by the U.S. Food and Drug Administration (FDA) for PsA: etanercept (a soluble fusion protein of TNF-α p75 receptor domains and an immunoglobulin (Ig)G Fc region), infliximab (a chimeric monoclonal anti–TNF-α antibody), and adalimumab (a fully human anti-TNF monoclonal antibody). These agents have been studied extensively in RA patients and demonstrate great clinical improvement in the signs and symptoms of inflammatory arthritis as well as improved functional status and quality of life.
The efficacy of etanercept has been confirmed in a large randomized, controlled trial of 205 PsA patients; improved America College of Rheumatology (ACR) clinical responses were seen at week 24.6 ACR responses are clinical composite response criteria developed by the ACR. For example, ACR 20 response would necessitate that a patient have a 20% reduction in the number of swollen and tender joints, and a reduction of 20% in three of the following five indices: physician global assessment of disease, patient global assessment of disease, pain, CRP/ESR, and HAQ. ACR 50 and ACR 70 require 50% and 70% reduction respectively in the same measures. The standard measure used in psoriasis—PASI75 (psoriasis activity and severity index, 75% improvement of a psoriatic lesion)—was achieved in 23% of subjects with etanercept compared with 3% with placebo.
The efficacy of infliximab has been confirmed in several studies. The largest of these is IMPACT II (Infliximab Multinational Psoriatic Arthritis Controlled Trial) with 200 PsA patients. Improved ACR clinical responses and PASI scores were improved in subjects in IMPACT.7 Adalimumab was also shown to be effective in PsA in one of the largest double-blind, placebo-controlled phase III trials, studying 313 PsA patients using ACR20/50/70 and PASI scores.8
Overall, anti-TNF therapy has been most effective in alleviating symptoms of axial disease, which methotrexate and sulfasalazine have been less likely to mitigate. Other biologic agents have been studied for PsA but were found to be more efficacious for the skin psoriasis than for the joint disease, including alefacept (fully humanized fusion protein, which binds to CD2 on memory T cells) and efalizumab (humanized antibody to the CD11 subunit of lymphocyte function-associated antigen 1 [LFA-1]).
The safety of anti-TNF agents has been studied in the above-mentioned clinical trials as well as postmarketing surveillance databases in both RA and PsA populations. Common adverse events of these agents include injection site reactions (usually self-limiting), increases upper respiratory illness, and, less commonly, a lupus-like syndrome. Additional rare but significant adverse effects have been found including the development of serious opportunistic infections such as tuberculosis. There have also been reports of new demyelinating disease, blood disorders, and the initiation and relapse of lymphoma. These adverse side effects must be carefully weighed against the potential benefits of slower disease progression in each patient.
Treatment with anti-TNF inhibitors has been a remarkable breakthrough in the treatment of PsA. In addition to improvement in the peripheral joint disease and skin psoriasis, there is a significant benefit in alleviating symptoms of axial disease, dactylitis, and enthesitis compared to traditional therapy. Because these agents have been highly successful in both skin and joint disease, it is important to build collaborative approaches with both rheumatologic and dermatologic specialties to optimize management of this disease.
Despite the success of biologic response modifiers, a limited number of patients do not respond to this treatment. Thus, additional targets are being studied where higher standards of clinical remission or arresting radiographic progression may be achieved. In certain cases, surgical options may be needed to correct severe joint destruction with joint replacement surgery.
Summary
- Presentation is heterogeneous; PsA is easily confused with rheumatoid arthritis or gout.
- Disease can affect both the skin and joints but not necessarily at the same time.
- It can affect any joint in the body.
- Up to 40% of patients can have a positive family history of psoriasis or arthritis.
- Up to 20% of patients with psoriasis develop PsA.
- There is no definitive laboratory or x-ray evidence for definitive diagnosis.
Suggested Readings
- Gelfand JM, Gladman DD, Mease PJ, et al: Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005;53(4):573.
- Gladman DD: Mortality in psoriatic arthritis. Clin Exp Rheumatol 2008;26(5 Suppl 5):S62-S65.
- Gladman DD, Mease PJ, Strand V, et al: Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34(5):1167-1170.
- Mease P: Current treatment for psoriatic arthritis and other spondyloarthritides. Rheum Dis Clin North Am. 2006;32(Suppl 1):11-20.
- Mease P: Psoriatic arthritis therapy. Curr Opin Rheumatol 2005;17:426-432.
- Qureshi AA, Dominguez P, Duffin KC, et al: Psoriatic arthritis screening tools. J Rheumatol 2008;35(7):1423-1425.
References
- Gladman DD, Antoni C, Mease P, et al: Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 Suppl 2:ii14-ii17.
- Gladman DD, Anhorn KA, Schachter RK, Mervart H: HLA antigens in psoriatic arthritis. J Rheumatol 1986;13(3):586-592.
- Olivieri I, Padula A, D’Angelo S, Scarpa R: Role of trauma in psoriatic arthritis. J Rheumatol 2008;35(11):2085-2087.
- Clark DW, Coulter DM: Psoriasis associated with rofecoxib. Arch Dermatol 2003;139(9):1223.
- Sarzi-Puttini P, Santandrea S, Boccassini L, et al: The role of NSAIDs in psoriatic arthritis: Evidence from a controlled study with nimesulide. Clin Exp Rheumatol 2001;19(1 Suppl 22):S17-S20.
- Mease PJ, Goffe BS, Metz J, et al: Etanercept in the treatment of psoriatic arthritis and psoriasis: A randomised trial. Lancet 2000;356(9227): 385-390.
- Antoni C, Krueger GG, de Vlam K, et al; IMPACT 2 Trial Investigators: Infliximab improves signs and symptoms of psoriatic arthritis: Results of the IMPACT 2 trial. Ann Rheum Dis 2005;64(8):1150-1157.
- Mease PJ, Gladman DD, Ritchlin CT, et al; Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group: Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52(10):3279-3289.