The lecture below can be accessed on the Disease Management section of the Cleveland Clinic, under Allergy and Immunology (to go to this link and see others in the series, please click here)
Atrial Fibrillation
Daniel J. Cantillon MD
Published: January 2014
Definition
Atrial fibrillation (AF) is a common heart rhythm disorder in which the electrical impulses in the upper cardiac chambers (atria) degenerate from their usual organized rhythm into a rapid chaotic pattern. This disruption results in an irregular and often rapid heartbeat that is classically described as “irregularly irregular” and occurs because of the unpredictable conduction of these disordered impulses across the atrioventricular (AV) node into the lower cardiac chambers (ventricles).
The definition and classification has been described and updated in 2011 guidelines published by the American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC), with the collaboration of the Heart Rhythm Society (HRS).1 According to these guidelines, an episode of AF is defined as an event lasting greater than 30 seconds in duration. Paroxysmal AF refers to patients with spontaneous termination of the arrhythmia within 7 days of its onset. Most patients spontaneously going in and out of AF on their own fall into this category. Persistent AF refers to patients with sustained arrhythmia beyond 7 days. Most patients in this category require the use of a therapeutic intervention to restore normal sinus rhythm. Long-standing persistent AF refers to patients with uninterrupted AF for more than 1 year. Permanent AF refers to patients in which efforts to restore normal sinus rhythm have either failed or been forgone. These categories are not mutually exclusive and it is common for patients with one type of AF to exhibit overlapping features of another type. In particular, the distinction between ‘long-standing persistent’ and ‘permanent’ AF largely reflect a treatment intention to restore normal sinus rhythm, or accept AF respectively among patients who have been in AF for more than 1 year.
Prevalence
AF is the most common sustained cardiac tachyarrhythmia encountered by clinicians in the United States and world-wide. It occurs in approximately 0.4% to 1.0% of the general population and affected 3 million Americans in 2005 with projections to reach nearly 8 million by 2050. Its prevalence increases with age, and it has been diagnosed at some point in up to 10% of the population older than 80 years. Thus, experts agree that the prevalence of AF will continue to grow, in particular, in the United States and other western countries with aging population demographics. This growth may also be influenced by extended survival outcomes for patients with congestive heart failure (CHF), valvular heart disease and coronary artery disease as AF is common among patients with other forms of structural heart disease.1
Pathophysiology
AF may be acutely associated with physiologic stressors such as surgical procedures, pulmonary embolism, chronic lung diseases, hyperthyroidism, and alcohol ingestion. Disease states commonly associated with AF include hypertension, valvular heart disease, CHF, coronary artery disease, Wolff-Parkinson-White (WPW) syndrome, pericarditis, obstructive sleep apnea and cardiomyopathy. Lone AF refers to patients without overt structural heart disease, or identifiable risk factors. Considerable research had been devoted to the disease mechanisms and pathogenesis, and this remains an area of active investigation. Achieving a complete understanding is limited by the complexity of this disorder and the heterogeneous patient population it affects.
Pathogenesis can be broadly divided into the categories of triggers, substrate and sustaining mechanisms. Since the late 1990s, it has been recognized that the initiation of AF in most cases occurs because of premature atrial contractions triggered by beats that arise from the pulmonary veins (PVs), usually from muscular tissue sleeves near the junction with the left atrium.2 These triggers may also fire repetitively and contribute to the maintenance of AF, essentially becoming drivers of AF. Up to a third of patients also demonstrate focal triggers originating outside the PVs. Focal triggers, especially the PVs, are felt to be very important early in the disease process and, in particular, among patients with paroxysmal AF. Over time, myocardial fibrosis develops within the atrial tissue in association with AF to support its maintenance by shortening affected tissue refractory periods. This has led to the long-held adage that ‘AF begets AF.’ The multiple wavelet model has suggested that AF is sustained by multiple simultaneous wavelets meandering throughout the atria. Atrial tissue with abnormal electrical propagation recorded by mapping catheters has been referred to as complex fractionated electrograms (CFEs). Expression of specific connecting protein channels at the cellular level are also felt to be important contributors to the disease substrate and sustaining mechanisms. Contemporary understanding of the AF substrate and sustaining mechanisms now also includes the role of the autonomic nervous system and, more recently, the discovery and evaluation of the concept of AF rotors.3,4
Cardiac ganglionic plexuses clustered posteriorly and superiorly to the left atrium are known to play an important role in the initiation and maintenance of AF. Evidence supportive of this concept includes therapeutic benefit derived from destruction of cardiac gangionic plexuses and also non-cardiac plexuses including the stellate ganglion and peri-nephric ganglia associated with the renal arteries.5 In addition, completely vagally denervated hearts, such in the case of cardiac transplantation, are also known to have a very low incidence of AF.6
AF rotors represent an emerging concept as a sustaining mechanism for AF involving spiral waves detected by spectral analysis of dominant frequencies recorded by intra-cardiac mapping catheters. Such spiral waves can be conceptualized as rotational wavelets breaking around a central localized source that could be either structural (i.e., scar-related) or purely functional (i.e., conduction heterogeneity involving certain cellular sodium and potassium channels). Novel methods to map and identify AF rotors have emerged.
AF may have hemodynamic consequences. It can decrease cardiac output by as much as 20% attributable to the contribution of atrial systole, and increase pulmonary capillary wedge pressure resulting in CHF. Deleterious hemodynamic effects also include non-physiologic tachycardia, increased valvular regurgitation, and irregularity in ventricular systole.
AF is associated with morbidity and even mortality. AF can produce bothersome symptoms that affect quality of life, but patients with AF also have a substantial risk of thromboembolic stroke, as discussed later. Some data demonstrate an association of AF with reduced overall survival.7
Signs and Symptoms
The clinical manifestation of AF is variable. Often, the symptoms are attributable to the rapid ventricular response. However, even when the ventricular response is controlled, symptoms can occur from loss of AV synchrony and/or atrial systole. This is particularly important for patients with left ventricular dysfunction (i.e., CHF). That said, some patients with AF are genuinely asymptomatic, even at rapid heart rates for unclear reasons. More often, however, patients report nonspecific symptoms such as fatigue, dyspnea, dizziness, and diaphoresis. Palpitations are a common feature. Occasionally, patients present with extreme manifestations of hemodynamic compromise, such as chest pain, pulmonary edema, or syncope. AF is present in 10% to 40% of patients with a new thromboembolic stroke.
Diagnosis
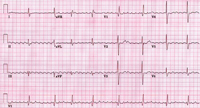
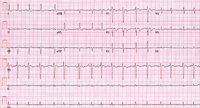
The clinician must realize that an irregular pulse detected by physical examination or an irregular ventricular rhythm seen on the electrocardiogram (ECG) is not always AF. It is necessary to consider and exclude other types of irregular rhythm disturbances, including atrial or ventricular ectopy, atrial tachycardia or atrial flutter (Figure 1) with variable AV conduction, multifocal atrial tachycardia (Figure 2), and wandering atrial pacemaker. Conversely, a regular pulse or rhythm does not exclude AF. For example, AF can manifest with a regular ventricular response in the presence of AV block or with a ventricular paced rhythm.
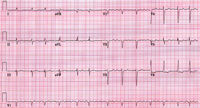
An ECG is essential for proper diagnosis. Electrocardiographic findings in AF include the absence of P waves, the presence of low amplitude, high frequency atrial fibrillary waves (f waves). The atrial rate varies in the range of 300 to 700 beats/min. In the absence of drug therapy, a patient with normal AV conduction has an irregularly irregular ventricular rhythm and often has a ventricular rate in the range of 120 to 180 beats/min. The baseline on the ECG strip often is undulating and occasionally has coarse irregular activity (Figure 3). This activity may resemble atrial flutter, but it is not as uniform from wave to wave as atrial flutter.
Treatment
Most patients presenting with AF are not in critical condition. However, in some cases, the presence of AF may be life threatening. It should be emphasized that for any unstable patient presenting with AF—for example, a patient with chest pain, pulmonary edema, or hypotension—the recommended therapy is rapid electrical cardioversion.
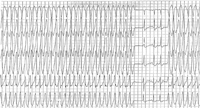
AF has particular importance in the setting of the WPW syndrome. Patients with WPW syndrome may be vulnerable to ventricular fibrillation and sudden death because of the development of AF, which can result in extremely rapid conduction over the accessory pathway (Figure 4). Prompt electrical cardioversion is of utmost importance for these patients. Treatment with AV node-blocking medications such as verapamil or digoxin can facilitate rapid conduction over the accessory pathway and result in ventricular fibrillation. When intravenous (IV) pharmacologic therapy is required, the drug of choice is procainamide or amiodarone.
The management of AF is directed at three basic goals: control of the ventricular rate, minimization of thromboembolism risk (particularly stroke), and restoration and maintenance of sinus rhythm. The first two management goals are essential for most patients, but the third management goal may not be necessary in all patients. The ACC/AHA/HRS guidelines provide a more detailed review of the management of AF with respect to which patients are treated purely with a rate-controlling approach, and which patients are selected for treatment designed to restore and maintain normal sinus rhythm (i.e., a rhythm controlling strategy).1
Control of the Ventricular Rate
The ventricular rate during AF may be rapid. Slowing is accomplished with medications affecting the AV node (Table 1). If these medications are ineffective or their effectiveness is prohibited by the development of excessive bradycardia, then other measures may need to be considered. One option suitable for some patients is catheter ablation of the AV node and pacemaker implantation (so-called ‘ablate and pace’). Meta-analysis of studies involving the ablate-and-pace approach8 has shown demonstrated improvements in a number of clinical parameters, including symptoms, quality of life, exercise function, cardiac performance and even longevity among patients with CHF receiving a bi-ventricular pacemaker. However, this approach usually results in pacemaker dependence. These patients may be exposed to the risks and complications of the implanted hardware. Pacemaker implantation without AV nodal ablation should be considered if the problem is simply excessive bradycardia that prohibits the effectiveness of rate-controlling medication. Strategies for suppression or cure of AF should be considered for appropriate patients before pursuing ablation of the AV node.
Table 1: Atrial Fibrillation Medications that Slow Conduction Through the Atrioventricular Node
| Drug | Advantages | Disadvantages | Usual Dosage | Onset of Action | Elimination Half-Life |
|---|---|---|---|---|---|
| Beta Blockers | |||||
| Propranolol | Rapid onset of effect, short durations of effect for IV forms; heart rate control at rest and with activity; oral forms available with varying durations of effect | May worsen heart failure in decompensated patient; may exacerbate reactive airway diseases; may cause fatigue, depression; abrupt withdrawal may cause rebound tachycardia, hypertension |
|
|
|
| Metoprolol |
|
|
|
||
| Atenolol |
|
|
|
||
| Esmolol (IV only) | IV: 500 µg/kg over 1 min, then maintenance dose of 25-300 µg/kg/min; titrate by 25-50 µg/kg/min q5-10min to achieve goal | IV: onset of action within 5 min | N/A | ||
| Nadolol (oral only) | Oral: 40-80 mg daily initially; increase to 240-320 mg daily as needed to achieve goal; can be given once daily | Oral: onset of action within 1-2 hr | 14-24 hr | ||
| Calcium Channel Blockers | |||||
| Diltiazem | Same as for beta blockers | May worsen heart failure in decompensated patient; may cause fatigue; abrupt withdrawal may cause rebound tachycardia, hypertension |
|
|
5-7 hr |
| Verapamil | Same as for beta blockers | May worsen heart failure in decompensated patient; may cause fatigue; abrupt withdrawal may cause rebound tachycardia, hypertension |
|
|
5-12 hr |
| Other | |||||
| Digoxin | Can be used in patients with heart failure | Slow onset of action; poor control of heart rate with activity; narrow therapeutic margin; long duration of effect | IV loading dose of up to 1.0 mg in first 24 hr, with bolus of 0.25-0.5 mg IV push; then remainder in divided doses 16-8hr; maintenance oral dose, 0.125-0.25 mg qd |
|
36 hr |
Preventing Thromboembolism and Stroke Risk
AF carries a considerable risk for thromboembolism and stroke. The Framingham study has shown that during a follow-up period of 30 years, the annual risk of stroke among AF patients is 4.2%; patients with nonvalvular AF had a more than fivefold higher risk of stroke. In the Framingham study, even patients with lone AF had a much higher incidence of stroke than controls over a period of almost 30 years.9 The annual risk of stroke may be even higher (in patients with AF who have one or more of the following risk factors: age older than 65 years, female gender, diabetes mellitus, hypertension, heart failure, coronary artery disease, previous stroke, or transient ischemic attack. Individual stroke risk stratification can now be calculated for patients on the basis of the presence or absence of such risk factors (i.e., CHADS2 and CHADS2 VA2S2C risk scores).10,11 Up until recent years, antithrombotic therapy for AF has been limited to the oral vitamin K antagonist warfarin or the antiplatelet agents aspirin, dipyridamole and clopidogrel. Warfarin has been shown to reduce the annual average relative risk of stroke by 68%, whereas the reduction with aspirin ranges from 0% to 44% (mean, approximately 20%). The combination of warfarin with aspirin increases the bleeding risk. Warfarin is superior to aspirin and also the combination of aspirin and clopidogrel in stroke prevention.12 Aspirin and clopidogrel is superior to aspirin alone in stroke protection among patients that are warfarin ineligible, but is associated with greater bleeding risk.13
Practice guidelines have been published regarding the recommended form of antithrombotic therapy for patients with AF.1 In general, aspirin monotherapy is appropriate for patients without any of the above-reference stroke risk factors, or perhaps only one non-critical risk factor (i.e., patients with calculated CHADS2 scores of 0 or 1). Any patient with prior stroke/transient ischemic attack or more than one risk factor should be considered for full anticoagulation with warfarin unless contraindicated by bleeding or some other consideration. The goal of warfarin therapy for preventing stroke and thromboembolism from AF generally is an international normalized ratio (INR) between 2.0 and 3.0. In recent years, novel oral anticoagulant drugs have emerged including direct thrombin inhibitors such as dabigatran (Pradaxa), or Factor Xa inhibitors such as rivaroxaban (Xarelto) or apixaban (Eliquis). In clinical studies, these agents have generally proven themselves as equally effective, if not more effective, than warfarin but with lower bleeding rates with the added convenience of not requiring INR monitoring. However, these drugs have not yet been established in an extended period of post-FDA approval to rival decades of clinical experience associated with the use of warfarin. Furthermore, such agents must be very carefully and selectively prescribed given specific concerns for elevated bleeding risk associated with specific drug-drug interactions (i.e., dronedarone and dabigatran) and renal dysfunction/chronic kidney disease. For patients who have been in AF for more than 48 hours and are not adequately anticoagulated, electrical or pharmacologic cardioversion should be delayed until appropriate measures are taken to reduce the thromboembolic risk. There are two approaches for patients being considered for cardioversion of AF longer than a duration of 48 hours. The conventional approach is to administer warfarin to achieve an INR value between 2.0 and 3.0 for at least 3 to 4 weeks before electrical or pharmacologic cardioversion. The second approach is the transesophageal echocardiography (TEE)-guided method. In some cases, cardioversion cannot be postponed for 3 or 4 weeks; in other cases, the patient, clinician, or both may prefer an expedited approach to achieving sinus rhythm. In such cases, once a therapeutic level of anticoagulation has been achieved with warfarin or IV heparin, TEE may be performed to exclude the presence of an intracardiac thrombus. If no thrombus is seen, cardioversion may be performed. TEE can detect the presence of a thrombus in the left atrium, particularly in the left atrial appendage, which is poorly seen on transthoracic echocardiography. The TEE-guided approach has been validated in several small multicenter trials as well as in a large, randomized, multicenter trial known as the Assessment of Cardioversion Using Transesophageal Echocardiography (ACUTE) trial.14
Warfarin should be continued after cardioversion until sinus rhythm has been maintained for at least 4 weeks to allow the atrial transport mechanism to recover. If the cardioversion was performed using the TEE-guided approach with IV heparin as the method of anticoagulation, it is advisable to continue IV heparin until a therapeutic INR is achieved with warfarin. The decision to initiate and continue anticoagulation for AF shorter than a duration of 48 hours should be based on the presence of other risk factors for thromboembolism. It is worthwhile to note that the previously mentioned novel oral anticoagulant drugs (dabigatran, rivaroxaban and apixaban) have not been FDA approved for direct current cardioversion, and for other AF-related procedures. Any such use is strictly off-label at this time, yet increasingly utilized.
Nonpharmacologic methods of stroke prevention include surgical left atrial exclusion or percutaneous left atrial appendage occlusion. Such interventions have shown great promise, but remain an active area of investigation without a proven indication.
Restoration and Maintenance of Sinus Rhythm
The restoration and maintenance of sinus rhythm can be beneficial for patients with bothersome symptoms. However, management of patients with asymptomatic or minimally symptomatic AF has been controversial for many years. Selecting appropriate patients for a rhythm-controlling strategy are well articulated in the 2011 updated clinical practice guidelines. In addition to improving symptoms, the potential benefits of restoring and maintaining sinus rhythm include avoidance of the development of atrial cardiomyopathy from ongoing AF, improvement in heart failure and improved overall quality of life in some studies. A rhythm control strategy often requires the use of antiarrhythmic drugs that may have significant and even life-threatening side effects, and procedures that carry uncommon but potentially life-threatening or disabling complications. Some nonrandomized trials have reported an increase in mortality among patients who were on long-term antiarrhythmic therapy for AF, presumably from the proarrhythmic effects of the drugs. In addition, several randomized studies have compared the treatment strategies of ventricular rate control or rhythm control with restoration and maintenance of sinus rhythm, albeit in older patients (mean age, 65-70 years) with minimal or no symptoms during AF.
The largest study, the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, was a large multicenter randomized study that compared these two treatment strategies for patients with AF.9 Both treatment strategies used appropriate anticoagulation strategies according to established guidelines. This study has demonstrated that a rhythm-control strategy is no better than a ventricular rate control strategy with regard to quality of life, incidence of stroke, or mortality at a follow-up of approximately 5 years. A meta-analysis of five randomized controlled trials of rate control versus rhythm control strategy included more than 5,000 patients and demonstrated that a rate-control strategy is not inferior to a rhythm-control strategy for the patients studied.15 Studies comparing catheter ablation with antiarrhythmic drug therapy as an initial approach to AF control have yielded some promising results.16,17 A larger multi-center randomized trial called the Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) study is ongoing, and should yield further insight. For the most part, catheter ablation is considered a second-line treatment for patients who have failed medical anti-arrhythmic drug therapy, or those with drug intolerance. Acutely, restoration of sinus rhythm may be achieved with either pharmacologic or electrical cardioversion. It is important to remember that electrical and pharmacologic cardioversion are no different with regard to the risk of thromboembolic stroke. Therefore, the requirements for anticoagulation apply equally to either treatment strategy and are largely dictated by the patient-specific thromboembolic risk profile discussed previously.
Direct-Current Electrical Cardioversion
Electrical cardioversion is more effective than pharmacologic cardioversion. The acute success rate for electrical direct current cardioversion with a biphasic shock is approximately 95%. Direct-current cardioversion should be administered with the patient under deep sedation, with cardiac and hemodynamic monitoring, and in the presence of personnel skilled in airway management. The administration of an antiarrhythmic drug may promote more successful direct current cardioversion and subsequent maintenance of sinus rhythm. Similarly, it is reasonable to add an antiarrhythmic drug for any patient who develops an early AF recurrence after direct-current cardioversion and to consider a repeat attempt after the drug has been initiated and reaches steady state blood levels.
Pharmacologic Cardioversion
Rates of successful immediate cardioversion by pharmacologic means have ranged from 40% to 90%, with success more likely to come for patients with AF of shorter duration. Contemporary use of pharmacologic cardioversion in the United States now centers around non-elective scenarios such as the emergency department or ICU setting, and also stable outpatients treated with a unique type of rhythm control strategy referred to as a ‘pill-in-the-pocket’ approach. Elective pharmacologic cardioversion is uncommon in the United States given the superiority of a planned electrical cardioversion under sedation with appropriate airway management personnel on hand.
The only IV agents approved in the United States for immediate pharmacologic cardioversion of AF are procainamide, amiodarone, and ibutilide (Table 2). Of those agents, amiodarone is the most commonly used drug in the ER and ICU settings. The pill-in-the-pocket treatment approach may be useful for select outpatients in order to terminate recent-onset episodes of AF. This approach has the potential to reduce emergency department visits and hospitalizations, but must be carefully initiated and supervised.
Table 2: Agents for Immediate Pharmacologic Cardioversion of Atrial Fibrillation
| Drug | Advantages | Disadvantages | Guidelines for Dosing | Comments |
|---|---|---|---|---|
| Procainamide, N-acetyl procainamide (NAPA) | Rapid administration may cause hypotension; up to 10% of congestive heart failure patients experience worsened heart failure | 10-15 mg/kg given IV up to 50 mg/min, then maintenance drip at 2-4 mg/min | Elimination half-life-2-5 hr for procainamide, 6-8 hr for NAPA; blood levels of both procainamide and NAPA need to be followed to prevent toxicity, especially in the setting of renal or hepatic insufficiency, or both | Can achieve therapeutic levels quickly |
| Amiodarone | Can be used in patients with severe left ventricular dysfunction | Long-term use associated with many side effects-visual disturbances, tremors and other neurologic sequelae, hepatitis, pulmonary fibrosis, photosensitivity, skin discoloration, thyroid abnormalities, cardiac conduction disturbances | 150-300 mg given over 10-120 min, depending on tolerance of blood pressure; maintenance infusion (very expensive) at 0.5-1 mg/min | Half-life is extremely long (up to 120 days) |
| Ibutilide | Few extracardiac side effects; ease of use | Incidence of torsades de pointes higher than with procainamide or amiodarone | 1-mg IV bolus; can repeat after 10 min if no effect | Avoid use in patients with baseline prolongation of QT interval |
Antiarrhythmic Drug Therapy for Maintenance of Sinus Rhythm
A number of oral agents may be used for long-term maintenance of sinus rhythm for patients with AF (Table 3). Quinidine was once widely prescribed for AF, but its use has significantly decreased in recent years. In fact, all of the Class IA antiarrhythmic drugs—quinidine, procainamide, and disopyramide—have become less popular for the long-term treatment of AF. Other antiarrhythmic drugs, such as the Class IC agents flecainide and propafenone, have more favorable side effect profiles. However, the use of these medications does have some degree of risk. The Cardiac Arrhythmia Suppression Trial (CAST) has shown that flecainide and encainide are associated with an increase in mortality when used for the suppression of ventricular arrhythmias in post-MI patients with ventricular dysfunction.18 As a result, there is much concern about the use of the Class IC antiarrhythmics in patients who have any type of underlying coronary artery or structural heart disease; in these patients, other antiarrhythmic drugs may be better initial choices. Flecainide and propafenone are usually well tolerated and are appropriate first-line options for the treatment of AF in patients without structural heart disease, particularly cardiomyopathy, including hypertrophic cardiomyopathy. There is a sustained-release formulation of propafenone that offers the advantage of twice-daily dosing rather than thrice-daily dosing, as for the immediate-release formulation.
Table 3: Oral Medications for Long-Term Maintenance of Sinus Rhythm for Patients With Atrial Fibrillation
| Drug | Advantages | Disadvantages | Guidelines for Dosing | Comments |
|---|---|---|---|---|
| Quinidine | Low cost; less negative inotropic effects | Low toxic-to-therapeutic ratio; interacts with many drugs, including digoxin, warfarin, verapamil; high incidence of side effects (particularly GI intolerance, neurologic side effects, hematologic suppressive effects) |
|
|
| Procainamide, N-acetyl procainamide (NAPA) | Useful in patients with pre-excitation and AF; prolongs refractory period of accessory pathway | Low toxic-to-therapeutic ratio; high incidence of GI, hematologic, immunologic (lupus-like syndrome) side effects; interacts with many medications |
|
|
| Flecainide | Generally well tolerated |
|
50-150 mg/dose q12hr |
|
| Propafenone (immediate-release, sustained-release) | Generally well tolerated; twice-daily dosing; sustained-release form results in more stable blood levels than immediate-release formulation |
|
|
Single-dose therapy with up to 600-mg doses shown to have high efficacy (˜75%) for converting acute episodes of AF to SR; cannot be used for acute cardioversion (pill-in-the-pocket) |
| Disopyramide | May be useful in hypertrophic cardiomyopathy because of its negative inotropic side effects | Anticholinergic effects (e.g., cardiomyopathy, constipation, urinary retention) | 150 mg/dose q6hr, 300 mg/dose q12hr for controlled-release preparation (give two thirds of usual dose for adults weighing <50 kg) | |
| Amiodarone | Can be used in patients with coronary or structural heart disease |
|
Oral loading with 400-600 mg/day for 2-4 wk, then 200 mg/day |
|
| Sotalol | Can be used in patients with coronary structural heart disease; beta- blocking properties allow single-agent therapy for almost all arrhythmias | Causes QTc prolongation; use limited by side effects related to beta-blocking properties (e.g., exacerbation of reactive airway disease, depression, negative inotropy) | 80-160 mg/dose q12hr | Inpatient telemetry recommended for initiation of therapy |
| Dofetilide | Generally well tolerated; few extracardiac effects; can be used in patients with coronary or structural heart disease | Causes QTc prolongation | 125-500 µg/dose q12hr | Inpatient telemetry mandated for initiation of therapy; ECG must be checked within 2-3 hr of administration for evidence of QTc prolongation >15% above baseline or >500 msec (550 msec for patients with intraventricular conduction delay) |
AF, atrial fibrillation; AV, atrioventricular; ECG, electrocardiogram; GI, gastrointestinal; LFT, liver function test; MI, myocardial infarction; SR, sinus rhythm; TFT, thyroid function test
Sotalol is a Class III antiarrhythmic that has beta-blocking properties and is generally well tolerated. Patients may have difficulty tolerating the beta blocker side effects, such as fatigue, and there is a potential risk of excessive bradycardia. As with other Class III antiarrhythmic agents, sotalol causes QT prolongation and may result in ventricular proarrhythmia, such as torsades de pointes.
Dofetilide, another Class III agent, has good efficacy rates and carries the principle advantage of being one of the best tolerated anti-arrhythmic drugs in terms of daily side effect profile. Importantly, dofetilide has also been shown to be safe for patients with cardiomyopathy, CHF, and ischemic heart disease. Therefore, dofetilide may be considered as an alternative treatment option to amiodarone. Like sotalol, this drug causes QT prolongation that may result in ventricular proarrhythmia and rarely death if excessive and is restricted to patients without advanced renal disease. Its use has been restricted by the FDA to certified prescribers and requires monitored initiation in a hospital setting followed by structured outpatient follow-up. Unlike sotalol, however, it does not cause excessive bradycardia and thus can be administered to patients without concern for exacerbating pre-existing bradycardia. This agent does have many potentially lethal drug-to-drug interactions, including many commonly prescribed antibiotics and anti-hypertensive drugs. Despite all of these limitations and drawbacks, many patients enjoy improved AF control on this agent without the nuisance of the daily side effects that limit use of some of the other anti-arrhythmic drugs.
Amiodarone, although an effective antiarrhythmic agent, generally is reserved for patients with AF for whom other antiarrhythmic drugs have been contraindicated, ineffective, or poorly tolerated. This is primarily because amiodarone has potential time- and dose-dependent organ toxicities that can affect the liver, thyroid, and lungs and the eyes. It is recommended that baseline studies be performed at the time of initiating this drug. These tests include an ophthalmologic examination, pulmonary spirometry and diffusion capacity tests, and blood tests to assess liver and thyroid function. The blood tests are often repeated at regular intervals, approximately every 6 to 12 months, and the ophthalmologic examination should be performed yearly. Cumulative toxicity with amiodarone occurs at a rate of approximately 2% per year. Dronedarone is a newer anti-arrhythmic drug designed to function similarly to amiodarone but without the molecular iodine interface associated with some of the previously described amiodarone toxicities. Early enthusiasm for this drug, based on results from the initial studies, was later tempered by safety concerns and limitations. The biggest safety concern with this drug involves use in patients with CHF. It is contraindicated for patients with advanced (NYHA functional class IV failure) and was found to increase cardiovascular death rates when given to patients with permanent AF in the Permanent Atrial fibriLLAtion Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) study.19 This drug also has some GI side effects that can be partially mitigated in many patients by taking with food. It also has some important drug-to-drug interactions, including with the anticoagulant drug dabigatran. That said, it is a reasonable treatment option for patients without structural heart disease, or advanced liver disease with the principal advantage of not requiring hospital-based initiation like dofetilide. It has also been one of the most heavily studied anti-arrhythmic drugs on the market.
Implantable Devices
Implantable cardiac devices are also applied in the treatment of patients with AF. Certainly, there is a substantial incidence of sinus node and AV node dysfunction in the AF population requiring cardiac pacing. Pacemakers have several purposes, including bradycardia pacing support, ventricular response regularization, and AF suppression or termination. Clinical practice guidelines detail the recommended uses of implantable pacemakers and antitachycardia devices. Pacemakers may be implanted simply for pacing support in patients with post-AF conversion pauses, or symptomatic bradycardia while in AF. Sinus node dysfunction in association with AF is often referred to as tachycardia-bradycardia syndrome (tachy-brady for short). Sinus node dysfunction can be exacerbated by medications used to control AF, and the presence of a pacemaker may allow greater use or up-titration of rate-controlling and/or antiarrhythmic medications.
Pacemakers are also implanted in conjunction with catheter ablation of the AV node. This type of ablation is the ultimate method of ventricular rate control and is often reserved for patients with permanent or paroxysmal AF refractory to medical or ablative therapy. The potential benefits of this type of approach extend beyond simply controlling ventricular response, because there is evidence that regularization of the ventricular rhythm also confers hemodynamic or symptomatic benefits, particularly in the heart failure population in conjunction with the use of a bi-ventricular pacemaker. This approach has been shown to be effective and leads to improved quality of life for patients. However, this approach does not address the fibrillating atria, and such patients still require systemic anticoagulation for thromboembolism and stroke prevention.
Several features of pacemaker systems may be useful for patients with AF. A pacemaker that has the capability to change automatically from a dual-chamber to single-chamber pacing mode at the onset of an episode of AF – commonly referred to as mode switching – is essential for avoiding the rapid heart rate that might otherwise occur when the pacemaker responds to the sensing of the rapid atrial activity. Other AF suppression algorithms have shown mixed results with regard to effectiveness. Implantable atrial defibrillators have been developed, either as a stand-alone device or in combination with a ventricular defibrillator. However, the atrial defibrillator has not been widely accepted by patients or physicians. In general, patients have difficulty tolerating even the low-energy internal cardioversion shocks or frequent anti-tachycardia pacing sequences without the deep sedation provided during conventional external cardioversion.
Catheter Ablation
With the recognition of PVs as the source of the critical triggering beats of AF in most patients, a standard catheter ablation approach involves achieving electrical PV isolation (PVI). The end result of this procedure is that spontaneous electrical impulses originating from within any of the four PVs cannot propagate into the atrial body to initiate or ‘trigger’ AF. PVI is thus a stand-alone treatment approach, but has also been incorporated into larger ablative efforts aimed at non-PV triggers and also substrate modification. Non-PV triggers include other focal sources of spontaneous or induced atrial ectopy, and substrate modification includes mapping/ablation of CFEs, denervation of cardiac ganglionic plexuses and, most recently, mapping and ablation of AF rotors. Substrate modification and/or ablation of non-PV triggers are often incorporated into procedures for patients with persistent or long-standing persistent AF. Outcomes data suggest that PVI alone without substrate modification works best in patients with paroxysmal AF, and that patients with persistent AF may derive additional benefit from additional substrate-based approaches. Published efficacy rates ange from as low as 50% to as high as 90%. Refinement in techniques have resulted in a lower incidence of complications, notably PV stenosis, which used to be very common in the early era of catheter ablation. Experienced centers have reported high rates of successful AF ablation resulting in discontinuation of antiarrhythmic drug therapy.20,21 The ideal candidate is a patient with paroxysmal AF in the absence of structural heart disease.
In virtually all studies involving catheter ablation, efficacy rates are lower among patients with persistent AF and long-standing persistent AF. The degree of atrial myopathy, scar burden and co-morbidities may also influence outcomes. Cryoballoon catheter ablation represents an alternative to traditional RF energy ablation associated with similar outcomes for paroxysmal AF patients but with shorter procedure times and less radiation exposure. The trade-off is a published phrenic nerve palsy rate of 11% that can occasionally result in long-term and/or permanent injury to normal diaphragm (breathing muscle) function.22 Such complications are only rarely seen with traditional RF ablation. Furthermore, there are no prospective data to support cryoballoon use among patients with persistent AF as this technique is purely aimed at achieving PVI without significant substrate modification. For this reason, utilization of cryoablation appears to have plateaued, and traditional RF ablation remains the overall more commonly applied method.
Experienced centers, such as the Cleveland Clinic, have reported 1-year AF freedom rates between 75% and 80% off anti-arrhythmic drugs for patients with paroxysmal AF following a single ablation procedure, and 85% to 90% following a second catheter ablation procedure. Approximately 20% to 30% of patients require a second ablation for AF owing to either recovery in the PVs, the presence of non-PV triggers or advanced atrial substrate/myopathy. Outcomes for patients with persistent and long-standing persistent AF are lower than patients with paroxysmal disease with reported 1-year efficacy rates between 50% and 70% following a single procedure and 70% and 80% following a second procedure. Procedure-related complications are low but include serious events such as stroke (0.5%) and PV stenosis (1%), cardiac tamponade (1%), serious esophageal injury (<1%), and death (0.1%). More common complications are typically non-life threatening, include femoral vascular related complications (2%) requiring intervention and/or delaying hospital discharge. Procedures typically take 4 to 6 hours, involve the use of radiation X-ray, and have an expected hospital course of overnight observation with planned discharge the next day.
Surgical Approaches
The original Cox maze surgical procedure for the treatment of AF has substantially evolved from its initial form. In general, it involves a series of incisions or lesions in the atria. These are carefully placed to compartmentalize the atrial tissue to channel atrial activity and prevent the re-entry required for the maintenance of AF. To a certain extent, there has been a confluence with some of the lesions sets delivered during catheter ablation techniques. For example, achieving anatomic PVI is now considered standard with both approaches. Non-incisional lesions may be placed using bipolar radiofrequency, cryothermy, or microwave energy. Outcomes associated with surgical approaches are comparable with catheter ablation (reported higher in some series) and offer the advantage of concomitant exclusion of the left atrial appendage.23,24 However, surgical approaches are more invasive than catheter ablation and requires either a sternotomy or a thoracotomy, plus general anesthesia and a longer post-operative recovery period. The incidence of perioperative complications has been low, but perhaps greater than catheter ablation. There is a potential need for a permanent postoperative pacemaker in as many as 7% to 10% of cases. This may occur as a result of the procedure itself or underlying sinus node dysfunction. The invasiveness of this approach makes it a less-desirable option for patients with AF alone, but it might be attractive for patients undergoing cardiac surgery for another indication (e.g., valve replacement or coronary bypass surgery), or patients in whom there is a particularly strong indication for exclusion of the left atrial appendage (i.e., recurrent thrombus despite anti-thrombotic therapy). Surgical approaches have continued to become less invasive. Several centers have been using minimally invasive incisions and even thoracoscopic approaches with robotic equipment. Some surgical centers have adopted incorporation of electrophysiologic testing and even catheter-based techniques into the procedure (i.e., the so-called hybrid procedure).
Outcomes
Treating AF is centered on quality of life because it is not immediately life threatening in most instances provided that patients receive appropriate thromboembolic stroke prophylaxis. However, it is associated with morbidity and mortality. Follow-up data from the Framingham Heart Study25,26 and the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial7 have shown that AF is an independent predictor of increased mortality. It is not clear whether this higher risk is a reflection of the proarrhythmic complications of antiarrhythmic therapy, a failure to comply with prescribed medical therapy, or the presence of other factors such as stroke, worsening CHF, or unknown factors that were not recognized.
Future Directions
The prevalence of AF, already at epidemic proportions, is expected to continue to increase as the population ages and more patients with heart disease live longer. This is especially true for the heart-failure population. The rapid growth of catheter-based and surgical ablation procedural capability is promising and has already relieved many patients of the burden of AF and the side effects and toxicities of antiarrhythmic medications. However, these approaches are invasive and inherently destructive, with a small but important risk of serious complications. Additional refinements to procedure-based approaches are anticipated. Neuromodulation procedures, including renal denervation, and mapping/ablation of AF rotors are the latest such investigational refinements.
The most beneficial development might be the effective prevention of AF. Research into the underlying molecular and genetic causes of AF may lead to novel methods of disease prevention.
Summary
- Atrial fibrillation is the most common sustained tachyarrhythmia in the United States and world-wide.
- Therapy for atrial fibrillation is centered around three goals: minimize thromboembolic stroke risk, control ventricular rate, and control the atrial rhythm in selected patients (i.e., rhythm control strategy).
- Any unstable patient presenting with atrial fibrillation should undergo immediate electrical cardioversion. Elective cardioversions are safe and effective with attention to appropriate anticoagulation strategies, including a transesophageal echocardiogram when needed.
- Treatment strategies involving rate control only are comparable to rhythm-control strategies in terms of mortality rates, but many studies support quality of life benefits associated with a rhythm-control approach.
- Procedure-based treatment for atrial fibrillation should be considered for symptomatic patients refractory to standard therapies. Such procedures include catheter ablation, surgical approaches and implantable cardiac devices.
References
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published online ahead of print December 21, 2010]. J Am Coll Cardiol 2011; 57:223–242. doi:10.1016/j.jacc.2010.10.001
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339:659–666.
- Zhang Y, Wang Z, Zhang Y, et al. Efficacy of cardiac autonomic denervation for atrial fibrillation: a meta-analysis [published online ahead of print March 19, 2012]. J Cardiovasc Electrophysiol 2012; 23:592–600. doi:10.1111/j.1540-8167.2011.02270.x
- Narayan SM, Patel J, Mulpuru S, Krummen DE. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study [published online ahead of print March 28, 2012]. Heart Rhythm 2012; 9:1436–1439. doi:10.1016/j.hrthm.2012.03.055
- Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension [published online ahead of print September 5, 2012]. J Am Coll Cardiol 2012; 60:1163–1170. doi:10.1016/j.jacc.2012.05.036
- Khan M, Kalahasti V, Rajagopal V, et al. Incidence of atrial fibrillation in heart transplant patients: long-term follow-up. J Cardiovasc Electrophysiol 2006; 17:827–831.
- Wyse DG, Love JC, Yao Q, et al. Atrial fibrillation: a risk factor for increased mortality-an AVID registry analysis. J Interv Card Electrophysiol 2001; 5:267–273.
- Wood MA, Brown-Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation2000; 101:1138–1144.
- The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation [published online ahead of print September 17, 2009]. Chest 2010; 137:263–272. doi:10.1378/chest.09-1584
- The ACTIVE Writing Group on behalf of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation [published online ahead of print March 31, 2009]. N Engl J Med2009; 360:2066–2078. doi:10.1056/NEJMoa0901301
- Klein AL, Murray RD, Grimm RA. Role of transesophageal echocardiography-guided cardioversion of patients with atrial fibrillation. J Am Coll Cardiol 2001; 37:691–704.
- de Denus S, Sanoski CA, Carlsson J, Opolski G, Spinler SA. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med 2005; 165:258–262.
- Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012; 367:1587–1595.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation [published online ahead of print November 14, 2011; published correction appears in N Engl J Med 2012; 366:672]. N Engl J Med 2011; 365:2268–2276. doi:10.1056/NEJMoa1109867
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002; 105:1077–1081.
- Wazni O, Wilkoff B, Saliba W. Catheter ablation for atrial fibrillation. N Engl J Med 2011; 365:2296–2304.
- Packer DL, Kowal RC, Wheelan KR, et al; for the STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial [published online ahead of print March 21, 2013]. J Am Coll Cardiol 2013; 61:1713–1723. doi:10.1016/j.jacc.2012.11.064
- McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D III. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg 2000; 12:25–29.
- Schaff HV, Dearani JA, Daly RC, Orszulak TA, Danielson GK. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg2000; 12:30–37.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998; 98:946–952.
- Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation: 30-year follow-up in the Framingham Study. JAMA 1985; 254:3449–3453.