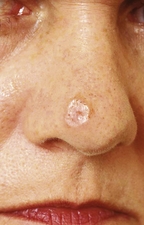
Melanoma
Rebecca Tung
Alison Vidimos
Published: August 2010
More than 1 million cases of skin cancer will be diagnosed in the United States this year. About 80% of these new skin cancer cases will be basal cell carcinoma, 16% will be squamous cell carcinoma, and 4% will be melanoma. It has been estimated that regular application of sunscreen with a sun protection factor of 15 or greater for the first 18 years of life would reduce the lifetime incidence of nonmelanoma skin cancers by 78%. Although nonmelanoma skin cancers (basal and squamous cell carcinomas) are the most common types of malignancies in humans, melanoma ranks as the sixth most common cancer.
Although the number of nonmelanoma skin cancers is staggering, they have a better than 95% cure rate if detected and treated early. Mortality is higher with melanoma: An estimated 8110 deaths resulted from melanoma in 2007. Because it has been shown that early detection has led to overall increased survival rates for melanoma patients, it is of utmost importance for all physicians to possess the clinical diagnostic skills necessary to identify early melanoma lesions and then refer patients for further appropriate evaluation and treatment.
Definition
Cutaneous malignant melanoma is a neoplasm arising from the melanocytes that can occur de novo or from a preexisting lesion such as a congenital, acquired, or atypical (dysplastic) nevus. Noncutaneous primary sites of melanocytes also include the mucosal epithelium, retinas, and leptomeninges. Because melanoma is potentially curable with surgical excision of early, thin lesions, prompt detection, diagnosis, and adequate removal of such lesions are of utmost importance. Education of the public with regard to the technique of routine self-examination and proper methods of sun protection can greatly improve the chances for early detection and adequate treatment of melanoma (Box 1). A multidisciplinary approach, including primary care physicians, dermatologists, surgeons, oncologists, immunologists, radiologists, pathologists, and epidemiologists is necessary to optimize detection and treatment of this increasingly common cancer.
| Box 1: American Academy of Dermatology Sun Safety Tips |
|---|
| Because overt exposure to ultraviolet light contributes to the formation of skin cancer, dermatologists recommend the following precautions. |
| Avoid peak sunlight hours—10 a.m. until 4 p.m.—when the sun’s rays are the strongest. |
Apply a broad-spectrum sunscreen, one that protects against UVA and UVB rays with SPF 15 or higher.
|
| Reapply sunscreen every 2 hours, especially after swimming or heavy perspiration. |
| Wear protective clothing including a wide-brimmed hat, sunglasses, long-sleeved shirt, and long pants. |
| Apply lip balm that contains sunscreen with SPF 15 or higher. |
| Seek shade while outdoors during the day. |
| Protect children by minimizing sun exposure and regularly applying sunscreen. This is crucial because excessive sun exposure in the first 18 years of life increases a person’s chances of developing melanoma. Eighty percent of lifetime sun exposure occurs before age 18 years. |
| Avoid reflective surfaces such as water, snow, and sand that can reflect up to 85% of the sun’s damaging rays. |
| Avoid tanning beds. |
SPF, sun protection factor; UVA, ultraviolet A; UVB, ultraviolet B.
Adapted from Sober AJ, Chuang TY, Duvic M, et al, for the Guidelines/Outcomes Committee. Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol 2001;45:579-586.
© 2002 The Cleveland Clinic Foundation.
Prevalence and risk factors
Recent U.S. incidence figures estimate that there were about 108, 230 new cases of melanoma in 2007: 48, 290 in situ (noninvasive) and 59, 940 invasive (33, 910 men and 26, 030 women). At current rates, 1 in 63 Americans will develop an invasive melanoma over a lifetime. One person dies of melanoma every hour. In 2007, about 8110 deaths were attributed to melanoma: 5220 men and 2890 women. Older white men have the highest mortality rates from melanoma. Melanoma is the sixth most common cancer in men and women. Melanoma is the second most common cancer in women ages 20 to 29 in the United States. The incidence of melanoma has increased 690% from 1950 to 2001. Apart from these statistics, if melanoma is detected and treated before it spreads, the 5-year survival rate is 99%.1,2
Pathophysiology
Evidence from epidemiologic studies shows that exposure to solar irradiation is the main cause of cutaneous melanoma in fair-complected persons.3, 4 This causal relation is supported by anatomic differences by sex, migration studies, difference in latitude of residence, and racial differences.
The most common site for melanoma in men is the upper back; in women, the most common sites are the lower legs and upper back.3 Studies have also shown that persons who immigrated to countries with higher levels of ambient solar radiation have increased rates of melanoma compared with similar people who did not move. Likewise, melanoma incidence and mortality rates in white persons were inversely correlated with distance from the equator. Racial differences also exist with respect to melanoma. The lower rate of melanoma in darkly pigmented persons results from the protective effect of melanin and smaller number of nevi that can serve as precursor lesions for melanoma. The main risk factors for cutaneous melanoma include phenotype (blue eyes, blond or red hair, and fair complexion), cutaneous reaction to sun exposure (freckling, inability to tan, sunburn tendency), history of severe (blistering) sunburns or intense intermittent sun exposures, upper socioeconomic status, family history of melanoma, number and subtypes of nevi (atypical nevi or giant melanocytic nevi), history of prior melanoma, and immunosuppression.3, 5
Genetic studies have also shown that 50% of familial melanomas and 25% of sporadic melanomas may be due to mutations in the tumor suppressor gene p16.6 Linkage studies have identified chromosome 9p21 as the familial melanoma gene.6 About 8% to 12% of all melanoma cases are familial melanoma. The familial melanoma syndrome (also known as the dysplastic nevus syndrome) has been defined as melanoma in one or more first- or second-degree relatives; large numbers of melanocytic nevi (often 50 to 100 or more), some of which are atypical and varied in size; and melanocytic nevi demonstrating certain histologic features. The mode of inheritance is most likely polygenic. The cumulative risk of developing cutaneous melanoma among persons with a history of familial melanoma is estimated to be approximately 50% by 50 years of age.6
Mutations in the gene CDKN2A within the 9p21 region have been demonstrated in familial melanoma kindreds. The CDKN2A gene is complex and codes for p16 and p14ARF, which both function to suppress cellular growth. An intact p16 inhibits cyclin-dependent kinases, a critical class of enzymes, whose function is to promote cellular proliferation by inhibiting the retinoblastoma protein. Therefore, an intact p16 is essential to arrest the cell cycle. p14ARF may be important in enhancing the effect of another tumor suppressor, p53.6
Five stages of tumor progression have been suggested:
- Benign melanocytic nevi
- Melanocytic nevi with architectural and cytologic atypia (dysplastic nevi)
- Primary malignant melanoma, radial growth phase
- Primary malignant melanoma, vertical growth phase
- Metastatic malignant melanoma
Each step in tumorigenesis is marked by a new clone of cells with growth advantages over the surrounding tissues.
Signs and symptoms
Early signs of melanoma include the ABCDEs: asymmetry of lesion; border irregularity, bleeding, or crusting; color change or variegation (some lesions are amelanotic [nonpigmented]); diameter larger than 6 mm or growing lesion; evolving (surface changes [raised, bleeding, crusting] or symptomatic [itchiness or tenderness]). About 1% to 2% of primary melanomas arise from mucous membrane melanocytes. Approximately 5% to 10% of patients present with metastatic disease (usually in the lymph node basin) without an identifiable primary lesion. Less than 2% of patients present with visceral metastases in the absence of an unknown primary lesion.
Precursor Lesions
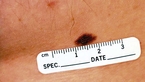
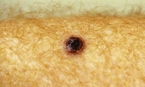
Acquired dysplastic nevi (Fig. 1) are atypical-appearing melanocytic tumors that are histologically characterized by intraepidermal melanocytic dysplasia. Dysplastic nevi are important because they are potential histogenic precursors of melanoma and markers of increased melanoma risk. Dysplastic nevi are fairly common; in the United States, 1.8% to 4.9% of white adults have dysplastic nevi. Dysplastic nevi start as rather large moles during the first decade of life. Almost 40% of children from families with dysplastic nevi melanoma have dysplastic nevi, and all children in whom melanoma eventually develops have dysplastic nevi. At least 17% of white adults with melanoma outside the familial melanoma setting have one or more dysplastic nevi, illustrating that dysplastic nevi are markers of risk, as well as potential precursors.
Clinically, dysplastic nevi appear by age 20 years as two or more disorderly distributed shades of brown and black. They may be round, oval, or misshapen with an irregular or fuzzy outline. Any site may be affected, even sun-protected sites. The horse-collar area is usually most heavily involved.7
Management for patients who have dysplastic nevi, with or without a personal or family history of melanoma, is controversial. Pathologic confirmation of the clinical diagnosis provides a more solid basis for making further management decisions. For people who have one or two suspected dysplastic nevi, excision is reasonable, but periodic examinations should be offered for a lifetime.7 Prophylactic removal of suspected dysplastic nevi is not feasible for people who have numerous dysplastic nevi. In patients with many dysplastic nevi, excision for hard-to-monitor areas (scalp, perineum, etc.) should be considered, and serial clinical photography of other lesions should be performed to detect new or changing lesions. Persons with dysplastic nevi should also be instructed on how to practice skin self-examination every 4 to 6 weeks at home.
For the removal of dysplastic nevi, lateral margins of about 2 to 3 mm should be taken to ensure complete removal.7 Dysplastic nevi can remain unchanged, progress to melanoma, or even regress over time. Only a small fraction of dysplastic nevi ever progress to melanoma, even in the familial melanoma setting. It is probable that both environmental and genetic factors play a role in the transition from dysplastic nevus to melanoma. In Greene and colleagues’ study of dysplastic nevi-melanoma kindreds, they found the actuarial probability of melanoma developing in persons who have dysplastic nevi in the familial melanoma setting may be as high as 56% from age 20 to 59 years and 100% by age 76 years.8
In summary, dysplastic nevi should be considered potential precursor lesions to melanoma and deserve careful surveillance and prompt treatment when required.
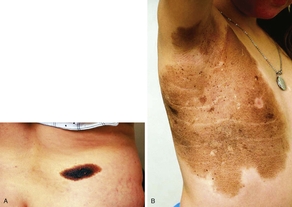
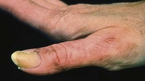
A short discussion on congenital nevi (Fig. 2) is presented here because patients often are concerned with the malignant potential of these lesions. A congenital nevus is defined as a melanocytic nevus that is present at birth or appears within the first few months of life. They are classified by size as small (<1.5 cm), medium (1.5-20.0 cm), and large (>20.0 cm). The risk of developing cutaneous melanoma within small- and medium-sized lesions is low but can be 1% over a lifetime. Conversely, large congenital nevi have an increased incidence of melanoma of up to 10% over a lifetime. Approximately 50% of the melanomas that develop within large congenital nevi do so by age 3 to 5 years, and patients have a melanoma risk of approximately 5% during the first 5 years of life.9 Therefore, smaller congenital nevi can be followed clinically, but early and complete surgical excision of large congenital nevi is usually recommended. If complete removal is not possible, the lesion should be closely observed and any nodules or suspicious changes should be biopsied.
Subtypes
The subtypes of melanoma are distinguished by clinical and pathologic growth patterns: superficial spreading, lentigo maligna, nodular, and acral lentiginous.
Lentigo Maligna and Lentigo Maligna Melanoma
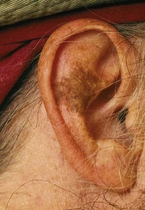
Lentigo maligna (melanoma in situ) (Fig. 3) begins as a tan irregular macule that extends peripherally, with differing shades throughout. It occurs on sun-damaged atrophic skin in elderly persons. Lentigo maligna occurs equally in men and women, usually in the seventh and eighth decades of life.7 The exact percentage of lentigo maligna that progress to invasive lentigo maligna melanoma is unknown, but it is estimated to be less than 30% to 50%. The lesion can grow slowly for 5 to 15 years in the precursor form before invasion.7 Although lentigo maligna has a prolonged radial growth phase, when invasion occurs, the result can be lethal. Long-term cumulative rather than intermittent sun exposure is believed to confer the greatest risk for developing lentigo maligna.
Lentigo maligna melanoma arises from lentigo maligna, a melanoma in situ (within the epidermis). Lentigo maligna melanoma is the least common subtype of melanoma, accounting for 4% to 15% of all melanoma patients.7 Lentigo maligna melanoma occurs almost exclusively on the sun-exposed skin of the head and neck; the nose and cheeks are the most common sites.10 Median age at diagnosis is 65 years. The lesion is usually quite large (3-6 cm or greater), with a variable nodular area from 1 mm to 2 cm in width.10 Rarely, lentigo maligna and lentigo maligna melanoma are amelanotic.
Superficial Spreading Melanoma
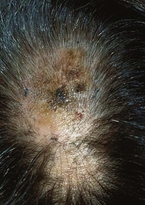
Superficial spreading melanoma (SSM) (Fig. 4) represents approximately 70% of all melanomas and is the most common type of cutaneous melanoma occurring in light-skinned people. It affects adults of all ages, with the peak incidence in the fourth and fifth decades of life. SSM, not uncommonly, can arise in a preexisting melanocytic nevus. The usual history is that of a slowly changing mole over 1 to 5 years.7 SSM most commonly affects intermittently sun-exposed areas with the greatest nevus density, such as the upper backs of men and women and lower legs of women.
Clinically, SSM starts as a deeply pigmented macule or plaque with intact skin markings. The earliest change in SSM can be a focal area of darkening within a preexisting nevus. Pigment variegation ranges from black and blue-gray to pink or gray-white. Absence of pigmentation within an SSM often represents regression of the melanoma, and the borders are often extremely irregular. The SSM subtype usually manifests with the classic early signs of melanoma (ABCDEs).
Nodular Melanoma
The second most common subtype of melanoma is nodular melanoma (Fig. 5). Nodular melanoma represents 15% of all melanomas. The median age at onset is 53 years. Clinically, nodular melanoma manifests as a uniform blue-black, blue-red, or amelanotic nodule. About 5% of nodular melanomas lack pigment (amelanotic melanoma). The most common sites for nodular melanoma are the trunk, head, and neck. It is more common for nodular melanoma to begin in normal skin rather than in a preexisting lesion. Rapid growth is also a hallmark of nodular melanoma.
Acral Lentiginous Melanoma
Acral lentiginous melanoma (Fig. 6) accounts for 10% of melanomas overall; however, they are the most common types among Japanese, African Americans, Latin Americans, and Native Americans. The median age for occurrence is 65 years, with equal gender distribution. The most common site of melanoma in African Americans is the feet, with 60% of patients having subungual or plantar lesions.7 Overall, acral lentiginous melanoma can occur on the palms or soles or beneath the nail plate; the sole is the most common site in all races. The average size at diagnosis is 3 cm, which may be related to delayed diagnosis. Clinically, the lesion is characterized by a tan, brown-to-black, flat macule with color variegation and irregular borders. Unlike lentigo maligna melanoma, development of acral lentiginous melanoma does not seem to be associated with sun exposure.
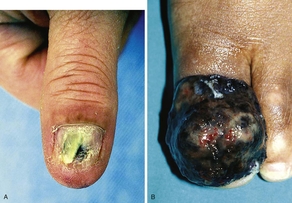
Subungual melanoma (Fig. 7) is a rare variant of acral lentiginous melanoma. Most subungual melanomas involve the great toe or thumb and generally arise from the nail matrix. Hutchinson’s sign is the finding of pigmentation on the posterior nail fold and is associated with advanced subungual melanoma.7
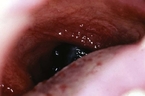
Other more uncommon variants of melanoma include melanoma of the mucosa (Fig. 8) and desmoplastic melanoma. When melanoma occurs on the mucosa, it usually develops on the mucosal surfaces of the head and neck (nasal and oral cavities), genital, or anorectal mucosa. Patients can present with bleeding or a mass lesion.
Desmoplastic Melanoma
Desmoplastic melanoma (Fig. 9) is a rare subtype of melanoma that is locally aggressive and has a high rate of local recurrence. It most commonly develops on sun-exposed skin of the head and neck of elderly persons in the sixth or seventh decade of life. Desmoplastic melanoma has a male predominance ratio of approximately 2 : 1. Approximately one half of desmoplastic melanomas develop in association with a lentigo maligna. Desmoplastic melanoma can manifest clinically as a pigmented macule with or without a nodular component or as a flesh-colored nodule without any surrounding pigmentation. Desmoplastic melanomas often invade perineurally and are, therefore, often symptomatic. Most desmoplastic melanomas are deeply invasive at the time of diagnosis, at least 5 to 6 mm thick. They have a propensity to recur and deeply invade locally.
Diagnosis
As with nonmelanoma skin cancers, biopsy is indicated for all suspicious pigmented lesions. Surface epiluminescence microscopy (dermatoscopy) and ultrasound are evolving adjunctive noninvasive diagnostic techniques.4 According to the American Academy of Dermatology (AAD) guidelines, whenever possible the lesion should be excised with narrow margins for diagnostic purposes. An incisional biopsy technique is appropriate when suspicion for melanoma is low, the lesion is large, or it is impractical to perform a complete excision. A repeat biopsy should be performed if the initial biopsy specimen is inadequate for accurate histologic diagnosis or staging. Fine needle aspiration cytology should not be used to assess the primary tumor. Histologic interpretation should be performed by a pathologist experienced in the microscopic diagnosis of pigmented lesions.4 The differential diagnosis is listed in Box 2.
| Box 2: Differential Diagnosis of Melanoma |
|---|
| Cherry angioma |
| Junctional or compound nevi |
| Kaposi’s sarcoma |
| Pigmented basal cell carcinoma |
| Pyogenic granuloma |
| Seborrheic keratosis |
Determining melanoma stage is important for planning appropriate treatment and assessing prognosis. The American Joint Commission on Cancer (AJCC) has revised the four-stage system, reflecting new findings that the Clark level (level of invasion according to depth of penetration of the dermis) offer little prognostic information for tumors thicker than 1 mm, whereas histologic ulceration consistently worsens prognosis across all tumors depths. There are now a and b (nonulcerated and ulcerated, respectively) categories for each primary tumor level, for a total of eight. A new stage, IIC, has been added, which represents clinically localized melanoma with the worst prognosis (thick, ulcerated primary tumors). The main changes of the revised AJCC staging system include simplified tumor-thickness thresholds from 0.75, 1.5, and 4.0 mm to 1.0, 2.0, and 4.0 mm; reassignment of thick tumors (>4.0 mm) from stage III to stage II; and elimination of the Clark level as a criterion except in tumors 1.0 mm thick. Additional staging criteria include presence or absence of microscopic ulceration, number of nodal metastases, metastatic burden, serum lactate dehydrogenase (LDH) level, and sentinel lymph node status.11
Eighty-five percent of melanoma patients have localized disease (stages I and II) on presentation. About 15% of patients have regional nodal disease, and only about 2% of patients have distant metastases at diagnosis. Prognosis for stages I and II melanoma can be affected by many factors. Factors associated with an improved prognosis include younger age, female gender, extremity lesions, and histologically negative nodes. Histologic variables associated with a less-favorable prognosis include increasing tumor thickness, deeper level of invasion, increased mitotic rate, ulceration, diminished lymphoid response, evidence of tumor regression, microscopic satellites, vascular invasion, and non–spindle-cell type tumors.
The presence of regional lymph node metastases imparts an overall 5-year survival rate of 37% and a 10-year survival of 32%.12 The most important prognostic factor for stage III melanoma is the number of positive lymph nodes. Patients with nodal micrometastases have an improved survival compared with patients with clinically palpable nodes. Patients with melanoma on an extremity and younger age at diagnosis have been shown to have a better prognosis. If there are distant metastases, median survival is about 6 to 9 months.13 For stage IV patients, the prognostic variables suggesting worse prognosis include increasing number of metastatic sites, visceral location of metastases (lung, liver, brain, bone), absence of resectable metastases, male gender, and shorter duration of remission.13 Patients with nonvisceral disease (e.g., skin, subcutaneous tissue, lymph nodes) have a better median survival, ranging from 12 to 15 months, and are more likely to respond to chemotherapy.13
Summary
- Thickness of lesion
- Presence or absence of ulceration
- Number of lymph nodes involved
- Size of lymph nodes
- Presence or absence of distant metastasis
Treatment
According to the AAD’s 2001 guidelines, surgical management of primary cutaneous melanoma should focus on obtaining an excision margin based on histologic confirmation of tumor-free margins.4
- Melanoma in situ: 0.5-cm margins
- Melanoma with Breslow’s thickness <2 mm: 1.0-cm margins
- Melanoma with Breslow’s thickness ≥2.0 mm: 2.0-cm margins
In certain circumstances, surgical management needs to be tailored to the individual case. Primary melanomas near a vital structure might require a reduced margin, and aggressive histologic features can suggest a more worrisome tumor and warrant a wider margin. Surgical excision at sites such as the fingers, toes, soles, and ears also need separate surgical considerations.
Mohs’ micrographic surgery might prove useful for excision of melanoma, especially lesions located on the head, neck, hands, and feet. However, there are no formal recommendations pending additional studies.4 Studies suggest that the current recommendation of 0.5-mm margins for lentigo maligna (melanoma in situ) is often insufficient. Mohs’ micrographic surgery and margin-controlled excision of lentigo maligna offer lower recurrence rates and allow tissue to be conserved.14, 15 Despite adequate surgical resection of the primary melanoma, approximately 15% to 36% of patients with stages I and II melanoma will have some form of recurrence or metastasis during their clinical course.
Routine laboratory tests and imaging studies are not required for asymptomatic patients with primary cutaneous melanoma 4 mm or less in thickness for initial staging or routine follow-up.4 Indications for such studies are directed by a thorough medical history and complete physical examination. However, some studies have suggested that a chest x-ray and serum lactate dehydrogenase (LDH) might help detect occult metastases and alter further clinical management.16, 17
Elective Lymph Node Dissection
Elective lymph node dissection is defined as removing regional lymph nodes that drain the site of the primary melanoma in the absence of any clinical evidence of nodal metastases. Elective lymph node dissection is a much-debated topic in the management of melanoma. Proponents have cited retrospective studies demonstrating improved prognosis for patients with intermediate thickness (1–4 mm) lesions.18-20 Opponents have cited prospective randomized trials that fail to show a statistically significant difference in survival rates following elective lymph node dissection.21-23
Sentinel Lymph Node Biopsy
Sentinel lymph node biopsy, a staging and possibly therapeutic procedure, is the most powerful predictor of melanoma recurrence and survival. Initially, lymphoscintigraphy is used to precisely map the draining nodal basin.24 The sentinel lymph node biopsy is based on the premise that the first node draining a lymphatic basin (sentinel lymph node) would be expected to predict the absence or presence of melanoma in that area.24 One percent isosulfan blue (Lymphazurin) dye is injected around the cutaneous lesion to allow intraoperative localization of this sentinel lymph node. Alternately, a radioactive tracer, technetium-99, can be injected at the lesion site. A gamma probe is used to pinpoint the radiolabeled lymph node, which is then removed for histopathologic review. If no melanoma cells are found, no further surgery is done. However, if the node does have melanoma cells, the remainder of the nodes in this area are removed.24
Determination of the status of the sentinel lymph node is relevant for several reasons. It has been shown to be an important independent prognostic factor: A positive result predicts high risk of treatment failure. It is a relatively low-risk procedure that can help identify high-risk patients who might benefit from additional therapy, such as selective complete lymphadenectomy or adjuvant interferon alfa-2b. It provides a psychological benefit for the patient whose sentinel lymph node biopsy does not reveal metastases.2 Because positivity rates for sentinel lymph node biopsy are less than 5% for AJCC T1 melanomas, sentinel lymph node biopsy is considered a low-yield procedure in most thin melanomas. The ideal Breslow criteria for selection of this technique are not yet established.2
Adjuvant Treatment with Interferon
In the Eastern Cooperative Oncology Group (ECOG) 1684 study, high-dose interferon alfa-2b was initially reported to improve the survival of patients with melanoma thicker than 4 mm; however, the follow-up trial ECOG 1690 did not show an overall survival benefit. Another major study reported by the Austrian Malignant Melanoma Cooperative Group did show that adjuvant treatment with low-dose interferon alfa-2b decreased the occurrence of metastases and prolonged the disease-free survival in patients with melanoma thicker than 1.5 mm. These studies suggest that the role of interferon in the treatment of melanoma is evolving and needs further study.
Chemotherapy
Systemic chemotherapy is primarily used in patients with advanced stage III (unresectable regional metastases) or stage IV (distant metastases) melanoma. Although most chemotherapy is not that effective, dacarbazine remains the most active drug and is the only FDA-approved chemotherapeutic agent for treating advanced melanoma in the United States.13 The response rate is in the range of 10% to 20%, and patients with metastases in the skin, subcutaneous tissues, or lymph nodes respond most often. Other combination chemotherapy and biochemotherapy regimens could achieve higher response rates but do not appear to lead to durable remission.13
Biologic Treatment
Therapy directed toward modulating or inducing the immune system against melanoma has gathered considerable interest in recent years. Interleukin-2 (IL-2) as a single agent has been used in metastatic melanoma. In one study, 7% of patients had a complete response, which was durable: Patients remained disease-free for up to 8 years after initiation of therapy.25 Another study also showed positive results in treating patients with their own tumor-infiltrating lymphocytes and IL-2. Monoclonal antibody therapies are still experimental and may be useful in melanoma. Likewise, melanoma vaccines have been developed to stimulate a specific response against melanoma-associated antigens. Vaccines are currently undergoing clinical trials.
Perfusion Chemotherapy
Isolated limb perfusion has been used for melanoma of the extremities. In isolated limb perfusion, the limb is isolated from the systemic circulation with a tourniquet, using arterial and venous cannulation; a chemotherapeutic agent is infused by means of a pump oxygenator, and then the medication is removed from the limb.26 It has been developed into the most effective method of treatment for local recurrent or in transit metastases of an extremity.26 Medications used for infusion include melphalan, dacarbazine, cisplatin, carboplatin, thiotepa, and cytokine tumor necrosis factor α.26
Radiation
Radiation therapy is used for palliation in certain patients with stage IV disease. Specific indications include brain metastases, pain associated with bone metastases, and superficial skin and subcutaneous metastases.
Prognosis
The prognosis for a patient with stage I or II melanoma is mainly related to tumor thickness7 (Table 1):
Table 1: American Joint Committee on Cancer 2002 Revised Melanoma Staging
| Overall Survival | ||||
|---|---|---|---|---|
|
|
||||
| Histologic Features | TNM Classification | 1-yr (%) | 5-yr (%) | 10-yr (%) |
| Stage 0 | ||||
| Intraepithelial or in situ melanoma | Tis N0 M0 | — | 100 | 100 |
| Stage I | ||||
| A | ||||
| ≤1 mm without ulceration and Clark level II/III | T1a N0 M0 | — | 95 | 88 |
| B | ||||
| ≤1 mm with ulceration or level IV/V | T1b N0 M0 | — | 91 | 83 |
| 1.01-2 mm without ulceration | T2a N0 M0 | — | 89 | 79 |
| Stage II | ||||
| A | ||||
| 1.01-2 mm with ulceration | T2b N0 M0 | — | 77 | 64 |
| 2.01–4 mm without ulceration | T3a N0 M0 | — | 79 | 64 |
| B | ||||
| 2.01–4 mm with ulceration | T3b N0 M0 | — | 63 | 51 |
| >4 mm without ulceration | T4a N0 M0 | — | 67 | 54 |
| C | ||||
| >4 mm with ulceration | T4b N0 M0 | — | 45 | 32 |
| Stage III | ||||
| A | ||||
| Single regional nodal micrometastasis, nonulcerated primary | T1-4a N1a M0 | — | 69 | 63 |
| 2–3 microscopic regional nodes, nonulcerated primary | T1-4a N2a M0 | — | 63 | 57 |
| B | ||||
| Single regional nodal micrometastasis, ulcerated primary | T1-4b N1a M0 | — | 53 | 38 |
| 2–3 microscopic regional nodes, ulcerated primary | T1-4b N2a M0 | — | 50 | 36 |
| Single regional nodal macrometastasis, nonulcerated primary | T1-4a N1b M0 | — | 59 | 48 |
| 2–3 macroscopic regional nodes, nonulcerated primary | T1-4a N2b M0 | — | 46 | 39 |
| In-transit metastases or satellite lesion(s) without metastatic lymph nodes | T1-4a/b N2c M0 | — | 30–50 | |
| C | ||||
| Single microscopic regional node, ulcerated primary | T1-4b N1b M0 | — | 29 | 24 |
| 2–3 macroscopic regional nodes, ulcerated primary | T1-4b N2b M0 | — | 24 | 15 |
| 4 or more metastatic nodes, matted nodes/gross extracapsular extension, or in-transit metastases ro satellite(s) and metastatic nodes | any T N3 M0 | — | 27 | 18 |
| Stage IV | ||||
| Distant skin, subcutaneous, or nodal metastases with normal LDH | any T; any N; M1a | 59 | 19 | 16 |
| Lung metastases with normal LDH | any T; any N; M1b | 57 | 7 | 3 |
| All other visceral metastases with normal LDH or any distant metastases with increased LDH | any T; any N; M1c | 41 | 9 | 6 |
Note: Thickness is defined as the thickness of the lesion using an ocular micrometer to measure the total vertical height of the melanoma from the granular layer to the area of deepest penetration. The Clark level refers to levels of invasion according to depth of penetration of the dermis.
LDH, lactate dehydrogenase; TNM, tumor, node, metastasis.
Adapted with permission from Balch CM, Buzald AC, Soong SJ, et al: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635-3548.
Data from Balch CM, Buzaid AC, Soong SJ, et al: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635-3648.
- Melanoma in situ: 100% survival at 5 years and 10 years
- Lesions ≤1 mm: 91%–95% at 5 years; 83%–88% at 10 years
- Lesions 1.01–2 mm: 77%–89% at 5 years; 64%–79% at 10 years
- Lesions 2.01–4 mm: 63%–79% at 5 years; 51%–64% at 10 years
- Lesions >4 mm: 45%–67% at 5 years; 32%–54% at 10 years
If grouped according to stage for localized primary melanoma, the overall survival rate is 80%.7 For patients with regional lymph node metastases (stage III disease), survival rates were 27% to 69% at 5 years and 18% to 63% at 10 years. Unfortunately, when there is evidence of distant metastases (stage IV disease), the 5-year survival rate is only 9% to 19%, and the 10-year survival rate is 6% to 16% (see Table 1). However, spontaneous regression has been documented in melanoma, even in patients with metastatic disease.7
Follow-up evaluation
The goal of regular follow-up evaluation of patients with melanoma is the detection of melanoma recurrence or development of a second primary melanoma. Each visit should include a detailed history and physical examination. For most patients with stage I or II melanoma, it is recommended that follow-up appointments be scheduled initially every 3 months for 2 years, then every 6 months for 3 years, then once yearly thereafter. If the patient has dysplastic nevi, the interval may be continued at every 6 months indefinitely. Photography may be helpful in following multiple clinically atypical nevi. Patients should also be taught and encouraged to practice monthly skin self-examination. Because most experts attribute the rising trend in the overall 5-year melanoma survival rate (some 40% in the 1940s to the current rate of 86%) to improved early detection, it is very important for both physicians and the public to be aware of the early warning signs of melanoma and to get appropriate dermatologic evaluation and treatment as soon as possible.
Prevention and screeening
Primary prevention of melanoma requires reduction of known risk factors in at-risk populations. The most important modifiable behavior for melanoma prevention is reduction of ultraviolet exposure. Education of the public regarding sun protection, risk factors for developing skin cancer, and skin self-examination is essential.27 The American Academy of Dermatology’s skin cancer awareness initiatives such as Melanoma Monday (an annual countrywide event offering free skin cancer screenings conducted by dermatologists to raise awareness about melanoma and encourage Americans to begin a lifelong habit of regular skin examinations) and Sun Smart programs have been particularly effective in creating positive change in society’s approach toward the sun.
References
- American Cancer Society. Statistics for 2009. PDFs available at www.cancer.org/docroot/stt/stt_0.asp (accessed March 2, 2009).
- American Academy of Dermatology. Melanoma Fact Sheet. Available at www.aad.org/media/background/factsheets/fact_melanoma.html (accessed March 2, 2009).
- Wagner JD, Gordon MS, Chuang TY, Coleman JJ 3rd. Current therapy of cutaneous melanoma. Plast Reconstr Surg. 2001, 105: 1774-1799.
- Sober AJ, Chuang TY, Duvic M, et al: for the Guidelines/Outcomes Committee: Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol. 2001, 45: 579-586.
- Cummins DL, Cummins JM, Pantle H, et al: Cutaneous malignant melanoma. Mayo Clinic Proc. 2006, 81: 500-507.
- Tsao H. Update on familial cancer syndromes and the skin. J Am Acad Dermatol. 2000, 42: 939-969.
- Odom RB, James WD, Berger TG. Melanocytic nevi and neoplasms. In: James WD, Berger TG, Elston D (eds): Andrews’ Diseases of the Skin. 9th ed. Philadelphia: WB Saunders, 2000, pp 881-889.
- Greene M, Clark WH Jr, Tucker MA, et al: High risk of malignant melanoma in melanoma-prone families with dysplastic nevi. Ann Intern Med. 1985, 102: 458-465.
- Egan CL, Oliveria SA, Elenitsas R, et al: Cutaneous melanoma risk and phenotypic changes in large congenital nevi: A follow-up study of 46 patients. J Am Acad Dermatol. 1998, 39: 923-932.
- Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 1995, 33: 923-936.
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004, 351: 998-1012.
- Buzzell RA, Zitelli JA. Favorable prognostic factors in recurrent and metastatic melanoma. J Am Acad Dermatol. 1996, 34: 798-803.
- Klimek VM, Wolchok JD, Chapman PB, et al: Systemic chemotherapy. Clin Plast Surg. 2000, 27: 451-461.
- Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005, 52: 92-100.
- Bub JL, Berg D, Slee A, Odland PB. Management of lentigo maligna and lentigo maligna melanoma with staged excision: A 5-year follow-up. Arch Dermatol. 2004, 140: 552-558.
- Khansur T, Sanders J, Das SK. Evaluation of staging workups in malignant melanoma. Arch Surg. 1989, 124: 847-849.
- Iscoe N, Kersey P, Gapski J, et al: Predictive value of staging patients with clinical stage I malignant melanoma. J. Plast Reconstr Surg. 1987, 80: 233-239.
- Milton GW, Shaw HM, McCarthy WH, et al: Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: Results of surgical treatment in 1, 319 patients. Br J Surg. 1982, 69: 108-111.
- Reintgen DS, Cox EB, McCarty KS, et al: Efficacy of elective lymph node dissections in patients with intermediate thickness primary melanoma. Ann Surg. 1983, 198: 379-385.
- Schneebaum S, Briele HA, Walker MJ, et al: Cutaneous thick melanoma. Prognosis and treatment. Arch Surg. 1987, 122: 707-711.
- Veronesi U, Adamus J, Bandiera DC, et al: Inefficacy of intermediate node dissection in stage I melanoma of the limbs. N Eng J Med. 1977, 297: 627-630.
- Veronesi U, Adamus J, Bandiera DC, et al: Delayed regional lymph node dissection in stage I melanoma of the lower extremities. Cancer. 1982, 49: 2420-2430.
- Sim FH, Taylor WF, Pritchard DJ, Soule EH. Lymphadenectomy in the management of stage I malignant melanoma: A prospective randomized study. Mayo Clin Proc. 1986, 61: 697-705.
- Ali-Salaam P, Ariyan S. Lymphatic mapping and sentinel lymph node biopsies. Clin Plast Surg. 2000, 27: 421-429.
- Rosenberg SA, Yang JC, Topalian SL, et al: Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994, 271: 907-913.
- Ma D, Ariyan S. The use of isolated limb perfusion to manage recurrent malignant melanoma. Clin Plast Surg. 2000, 27: 441-450.
- Markovic SN, Erickson LA, Rao RD, et al: Melanoma Study Group of the Mayo Clinic Cancer Center: Malignant melanoma in the 21st century, part 1: Epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007, 82: 364-380.